Abstract
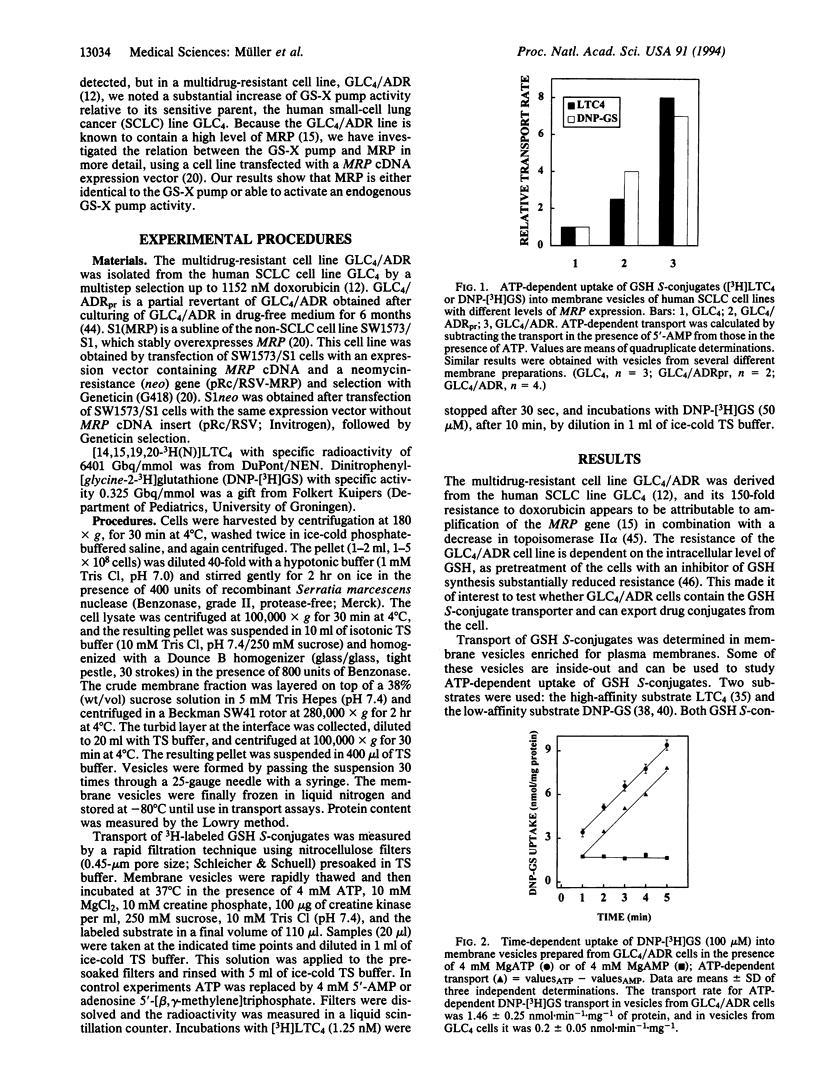
The multidrug resistance-associated protein (MRP) is a 180- to 195-kDa glycoprotein associated with multidrug resistance of human tumor cells. MRP is mainly located in the plasma membrane and it confers resistance by exporting natural product drugs out of the cell. Here we demonstrate that overexpression of the MRP gene in human cancer cells increases the ATP-dependent glutathione S-conjugate carrier activity in plasma membrane vesicles isolated from these cells. The glutathione S-conjugate export carrier is known to mediate excretion of bivalent anionic conjugates from mammalian cells and is thought to play a role in the elimination of conjugated xenobiotics. Our results suggest that MRP can cause multidrug resistance by promoting the export of drug modification products from cells and they shed light on the reported link between drug resistance and cellular glutathione and glutathione S-transferase levels.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerboom T. P., Narayanaswami V., Kunst M., Sies H. ATP-dependent S-(2,4-dinitrophenyl)glutathione transport in canalicular plasma membrane vesicles from rat liver. J Biol Chem. 1991 Jul 15;266(20):13147–13152. [PubMed] [Google Scholar]
- Andrews P. A., Brenner D. E., Chou F. T., Kubo H., Bachur N. R. Facile and definitive determination of human adriamycin and daunoribicin metabolites by high-pressure liquid chromatography. Drug Metab Dispos. 1980 May-Jun;8(3):152–156. [PubMed] [Google Scholar]
- Anundi I., Högberg J., Vahter M. GSH release in bile as influenced by arsenite. FEBS Lett. 1982 Aug 23;145(2):285–288. doi: 10.1016/0014-5793(82)80184-1. [DOI] [PubMed] [Google Scholar]
- Awasthi S., Singhal S. S., Srivastava S. K., Zimniak P., Bajpai K. K., Saxena M., Sharma R., Ziller S. A., 3rd, Frenkel E. P., Singh S. V. Adenosine triphosphate-dependent transport of doxorubicin, daunomycin, and vinblastine in human tissues by a mechanism distinct from the P-glycoprotein. J Clin Invest. 1994 Mar;93(3):958–965. doi: 10.1172/JCI117102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas F., Jongsma A. P., Broxterman H. J., Arceci R. J., Housman D., Scheffer G. L., Riethorst A., van Groenigen M., Nieuwint A. W., Joenje H. Non-P-glycoprotein mediated mechanism for multidrug resistance precedes P-glycoprotein expression during in vitro selection for doxorubicin resistance in a human lung cancer cell line. Cancer Res. 1990 Sep 1;50(17):5392–5398. [PubMed] [Google Scholar]
- Barrand M. A., Heppell-Parton A. C., Wright K. A., Rabbitts P. H., Twentyman P. R. A 190-kilodalton protein overexpressed in non-P-glycoprotein-containing multidrug-resistant cells and its relationship to the MRP gene. J Natl Cancer Inst. 1994 Jan 19;86(2):110–117. doi: 10.1093/jnci/86.2.110. [DOI] [PubMed] [Google Scholar]
- Berhane K., Widersten M., Engström A., Kozarich J. W., Mannervik B. Detoxication of base propenals and other alpha, beta-unsaturated aldehyde products of radical reactions and lipid peroxidation by human glutathione transferases. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1480–1484. doi: 10.1073/pnas.91.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black S. M., Beggs J. D., Hayes J. D., Bartoszek A., Muramatsu M., Sakai M., Wolf C. R. Expression of human glutathione S-transferases in Saccharomyces cerevisiae confers resistance to the anticancer drugs adriamycin and chlorambucil. Biochem J. 1990 Jun 1;268(2):309–315. doi: 10.1042/bj2680309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan H. L., Beverley S. M. Heavy metal resistance: a new role for P-glycoproteins in Leishmania. J Biol Chem. 1991 Oct 5;266(28):18427–18430. [PubMed] [Google Scholar]
- Chen C. J., Chin J. E., Ueda K., Clark D. P., Pastan I., Gottesman M. M., Roninson I. B. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986 Nov 7;47(3):381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- Cole S. P., Bhardwaj G., Gerlach J. H., Mackie J. E., Grant C. E., Almquist K. C., Stewart A. J., Kurz E. U., Duncan A. M., Deeley R. G. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992 Dec 4;258(5088):1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- Dano K. Active outward transport of daunomycin in resistant Ehrlich ascites tumor cells. Biochim Biophys Acta. 1973 Oct 25;323(3):466–483. doi: 10.1016/0005-2736(73)90191-0. [DOI] [PubMed] [Google Scholar]
- Delnomdedieu M., Basti M. M., Otvos J. D., Thomas D. J. Reduction and binding of arsenate and dimethylarsinate by glutathione: a magnetic resonance study. Chem Biol Interact. 1994 Feb;90(2):139–155. doi: 10.1016/0009-2797(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Detke S., Katakura K., Chang K. P. DNA amplification in arsenite-resistant Leishmania. Exp Cell Res. 1989 Jan;180(1):161–170. doi: 10.1016/0014-4827(89)90220-6. [DOI] [PubMed] [Google Scholar]
- Elferink R. P., Ottenhoff R., Liefting W., de Haan J., Jansen P. L. Hepatobiliary transport of glutathione and glutathione conjugate in rats with hereditary hyperbilirubinemia. J Clin Invest. 1989 Aug;84(2):476–483. doi: 10.1172/JCI114189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flens M. J., Izquierdo M. A., Scheffer G. L., Fritz J. M., Meijer C. J., Scheper R. J., Zaman G. J. Immunochemical detection of the multidrug resistance-associated protein MRP in human multidrug-resistant tumor cells by monoclonal antibodies. Cancer Res. 1994 Sep 1;54(17):4557–4563. [PubMed] [Google Scholar]
- Godwin A. K., Meister A., O'Dwyer P. J., Huang C. S., Hamilton T. C., Anderson M. E. High resistance to cisplatin in human ovarian cancer cell lines is associated with marked increase of glutathione synthesis. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3070–3074. doi: 10.1073/pnas.89.7.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. M. How cancer cells evade chemotherapy: sixteenth Richard and Hinda Rosenthal Foundation Award Lecture. Cancer Res. 1993 Feb 15;53(4):747–754. [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Grant C. E., Valdimarsson G., Hipfner D. R., Almquist K. C., Cole S. P., Deeley R. G. Overexpression of multidrug resistance-associated protein (MRP) increases resistance to natural product drugs. Cancer Res. 1994 Jan 15;54(2):357–361. [PubMed] [Google Scholar]
- Heijn M., Oude Elferink R. P., Jansen P. L. ATP-dependent multispecific organic anion transport system in rat erythrocyte membrane vesicles. Am J Physiol. 1992 Jan;262(1 Pt 1):C104–C110. doi: 10.1152/ajpcell.1992.262.1.C104. [DOI] [PubMed] [Google Scholar]
- Higgins C. F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- Hospers G. A., Mulder N. H., de Jong B., de Ley L., Uges D. R., Fichtinger-Schepman A. M., Scheper R. J., de Vries E. G. Characterization of a human small cell lung carcinoma cell line with acquired resistance to cis-diamminedichloroplatinum(II) in vitro. Cancer Res. 1988 Dec 1;48(23):6803–6807. [PubMed] [Google Scholar]
- Ishikawa T. ATP/Mg2+-dependent cardiac transport system for glutathione S-conjugates. A study using rat heart sarcolemma vesicles. J Biol Chem. 1989 Oct 15;264(29):17343–17348. [PubMed] [Google Scholar]
- Ishikawa T., Ali-Osman F. Glutathione-associated cis-diamminedichloroplatinum(II) metabolism and ATP-dependent efflux from leukemia cells. Molecular characterization of glutathione-platinum complex and its biological significance. J Biol Chem. 1993 Sep 25;268(27):20116–20125. [PubMed] [Google Scholar]
- Ishikawa T., Müller M., Klünemann C., Schaub T., Keppler D. ATP-dependent primary active transport of cysteinyl leukotrienes across liver canalicular membrane. Role of the ATP-dependent transport system for glutathione S-conjugates. J Biol Chem. 1990 Nov 5;265(31):19279–19286. [PubMed] [Google Scholar]
- Ishikawa T. The ATP-dependent glutathione S-conjugate export pump. Trends Biochem Sci. 1992 Nov;17(11):463–468. doi: 10.1016/0968-0004(92)90489-v. [DOI] [PubMed] [Google Scholar]
- Juliano R. L., Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976 Nov 11;455(1):152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- Kitamura T., Jansen P., Hardenbrook C., Kamimoto Y., Gatmaitan Z., Arias I. M. Defective ATP-dependent bile canalicular transport of organic anions in mutant (TR-) rats with conjugated hyperbilirubinemia. Proc Natl Acad Sci U S A. 1990 May;87(9):3557–3561. doi: 10.1073/pnas.87.9.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T., Dale G. L., Beutler E. Glutathione transport by inside-out vesicles from human erythrocytes. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6359–6362. doi: 10.1073/pnas.77.11.6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R. A., Zakher J., Kim G. Role of the glutathione redox cycle in acquired and de novo multidrug resistance. Science. 1988 Aug 5;241(4866):694–697. doi: 10.1126/science.3399900. [DOI] [PubMed] [Google Scholar]
- Krishnamachary N., Center M. S. The MRP gene associated with a non-P-glycoprotein multidrug resistance encodes a 190-kDa membrane bound glycoprotein. Cancer Res. 1993 Aug 15;53(16):3658–3661. [PubMed] [Google Scholar]
- Lankelma J., Spoelstra E. C., Dekker H., Broxterman H. J. Evidence for daunomycin efflux from multidrug-resistant 2780AD human ovarian carcinoma cells against a concentration gradient. Biochim Biophys Acta. 1990 Dec 10;1055(3):217–222. doi: 10.1016/0167-4889(90)90035-c. [DOI] [PubMed] [Google Scholar]
- Lee T. C., Wei M. L., Chang W. J., Ho I. C., Lo J. F., Jan K. Y., Huang H. Elevation of glutathione levels and glutathione S-transferase activity in arsenic-resistant Chinese hamster ovary cells. In Vitro Cell Dev Biol. 1989 May;25(5):442–448. doi: 10.1007/BF02624629. [DOI] [PubMed] [Google Scholar]
- Leier I., Jedlitschky G., Buchholz U., Keppler D. Characterization of the ATP-dependent leukotriene C4 export carrier in mastocytoma cells. Eur J Biochem. 1994 Mar 1;220(2):599–606. doi: 10.1111/j.1432-1033.1994.tb18661.x. [DOI] [PubMed] [Google Scholar]
- Leyland-Jones B. R., Townsend A. J., Tu C. P., Cowan K. H., Goldsmith M. E. Antineoplastic drug sensitivity of human MCF-7 breast cancer cells stably transfected with a human alpha class glutathione S-transferase gene. Cancer Res. 1991 Jan 15;51(2):587–594. [PubMed] [Google Scholar]
- Meijer C., Mulder N. H., Timmer-Bosscha H., Peters W. H., de Vries E. G. Combined in vitro modulation of adriamycin resistance. Int J Cancer. 1991 Oct 21;49(4):582–586. doi: 10.1002/ijc.2910490419. [DOI] [PubMed] [Google Scholar]
- Meijer C., Mulder N. H., Timmer-Bosscha H., Zijlstra J. G., de Vries E. G. Role of free radicals in an adriamycin-resistant human small cell lung cancer cell line. Cancer Res. 1987 Sep 1;47(17):4613–4617. [PubMed] [Google Scholar]
- Meister A. Glutathione deficiency produced by inhibition of its synthesis, and its reversal; applications in research and therapy. Pharmacol Ther. 1991;51(2):155–194. doi: 10.1016/0163-7258(91)90076-x. [DOI] [PubMed] [Google Scholar]
- Mirski S. E., Gerlach J. H., Cole S. P. Multidrug resistance in a human small cell lung cancer cell line selected in adriamycin. Cancer Res. 1987 May 15;47(10):2594–2598. [PubMed] [Google Scholar]
- Morrow C. S., Cowan K. H. Glutathione S-transferases and drug resistance. Cancer Cells. 1990 Jan;2(1):15–22. [PubMed] [Google Scholar]
- Müller M., Ishikawa T., Berger U., Klünemann C., Lucka L., Schreyer A., Kannicht C., Reutter W., Kurz G., Keppler D. ATP-dependent transport of taurocholate across the hepatocyte canalicular membrane mediated by a 110-kDa glycoprotein binding ATP and bile salt. J Biol Chem. 1991 Oct 5;266(28):18920–18926. [PubMed] [Google Scholar]
- Nooter K., Sonneveld P. Clinical relevance of P-glycoprotein expression in haematological malignancies. Leuk Res. 1994 Apr;18(4):233–243. doi: 10.1016/0145-2126(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Ouellette M., Fase-Fowler F., Borst P. The amplified H circle of methotrexate-resistant leishmania tarentolae contains a novel P-glycoprotein gene. EMBO J. 1990 Apr;9(4):1027–1033. doi: 10.1002/j.1460-2075.1990.tb08206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette M., Papadopoulou B. Mechanisms of drug resistance in Leishmania. Parasitol Today. 1993 May;9(5):150–153. doi: 10.1016/0169-4758(93)90135-3. [DOI] [PubMed] [Google Scholar]
- Papadopoulou B., Roy G., Dey S., Rosen B. P., Ouellette M. Contribution of the Leishmania P-glycoprotein-related gene ltpgpA to oxyanion resistance. J Biol Chem. 1994 Apr 22;269(16):11980–11986. [PubMed] [Google Scholar]
- Puchalski R. B., Fahl W. E. Expression of recombinant glutathione S-transferase pi, Ya, or Yb1 confers resistance to alkylating agents. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2443–2447. doi: 10.1073/pnas.87.7.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinkel A. H., Borst P. Multidrug resistance mediated by P-glycoproteins. Semin Cancer Biol. 1991 Aug;2(4):213–226. [PubMed] [Google Scholar]
- Scott N., Hatlelid K. M., MacKenzie N. E., Carter D. E. Reactions of arsenic(III) and arsenic(V) species with glutathione. Chem Res Toxicol. 1993 Jan-Feb;6(1):102–106. doi: 10.1021/tx00031a016. [DOI] [PubMed] [Google Scholar]
- Sikic B. I. Modulation of multidrug resistance: at the threshold. J Clin Oncol. 1993 Sep;11(9):1629–1635. doi: 10.1200/JCO.1993.11.9.1629. [DOI] [PubMed] [Google Scholar]
- Slovak M. L., Ho J. P., Bhardwaj G., Kurz E. U., Deeley R. G., Cole S. P. Localization of a novel multidrug resistance-associated gene in the HT1080/DR4 and H69AR human tumor cell lines. Cancer Res. 1993 Jul 15;53(14):3221–3225. [PubMed] [Google Scholar]
- Wang H. F., Lee T. C. Glutathione S-transferase pi facilitates the excretion of arsenic from arsenic-resistant Chinese hamster ovary cells. Biochem Biophys Res Commun. 1993 May 14;192(3):1093–1099. doi: 10.1006/bbrc.1993.1529. [DOI] [PubMed] [Google Scholar]
- Zaman G. J., Flens M. J., van Leusden M. R., de Haas M., Mülder H. S., Lankelma J., Pinedo H. M., Scheper R. J., Baas F., Broxterman H. J. The human multidrug resistance-associated protein MRP is a plasma membrane drug-efflux pump. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8822–8826. doi: 10.1073/pnas.91.19.8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman G. J., Versantvoort C. H., Smit J. J., Eijdems E. W., de Haas M., Smith A. J., Broxterman H. J., Mulder N. H., de Vries E. G., Baas F. Analysis of the expression of MRP, the gene for a new putative transmembrane drug transporter, in human multidrug resistant lung cancer cell lines. Cancer Res. 1993 Apr 15;53(8):1747–1750. [PubMed] [Google Scholar]
- Zijlstra J. G., de Vries E. G., Mulder N. H. Multifactorial drug resistance in an adriamycin-resistant human small cell lung carcinoma cell line. Cancer Res. 1987 Apr 1;47(7):1780–1784. [PubMed] [Google Scholar]
- de Jong S., Zijlstra J. G., de Vries E. G., Mulder N. H. Reduced DNA topoisomerase II activity and drug-induced DNA cleavage activity in an adriamycin-resistant human small cell lung carcinoma cell line. Cancer Res. 1990 Jan 15;50(2):304–309. [PubMed] [Google Scholar]