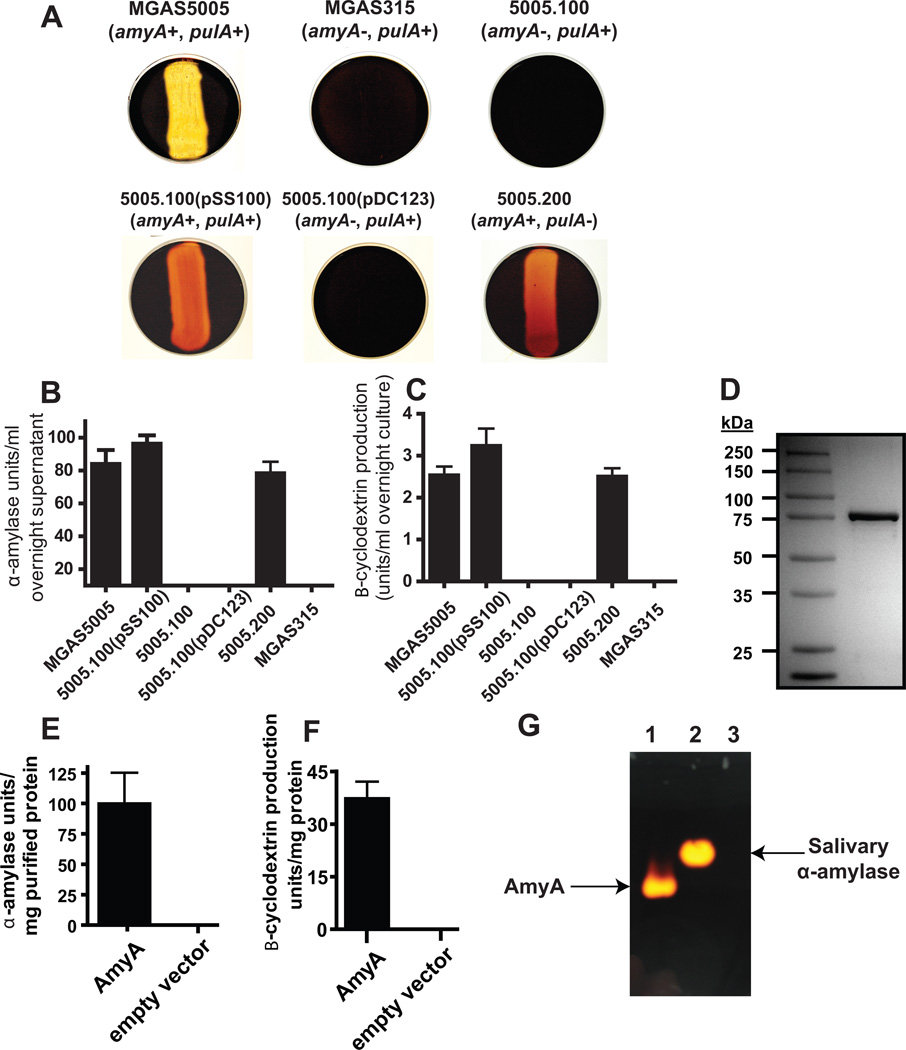
Fig. 2.
AmyA α-glucan digestion results in β-cyclomaltodextrin formation. (A) GAS strains of indicated amyA and pulA genotype were grown overnight on THY agar plates supplemented with 0.5% starch. Iodine was added to plates and the presence of clearing was assessed for evidence of starch hydrolysis. (B) Colorimetric analysis of iodine staining following growth in THY supplemented with 1% starch was used to assess starch degrading activity in indicated GAS strains as described in Experimental procedures. (C) β-cyclomaltodextrin production from starch by various strains was assessed using colorimetric analysis as described in Experimental procedures. (D) GAS AmyA was cloned and overexpressed in E. coli and then purified to apparent homogeneity as described in Experimental procedures. Proteins were separated on a 12% polyacrylamide gel. (E) Degradation of starch by purified AmyA with empty pBAD-hisA vector as negative control. (F) Production of B-cyclomaltodextrin by purified AmyA with empty pBAD-hisA vector as negative control. (G) Purified AmyA was run under non-denaturing conditions as described in Experimental procedures and subsequently allowed to diffuse into a 1% starch agar plate. Iodine staining confirmed that starch degradation (bright areas) was limited to the gel locations corresponding to AmyA. Lanes are as follows; 1 = 5 µl purified AmyA, 2 = 5 μl human salivary α-amylase, 3 = 5 µl empty vector. All starch degrading experiments were performed in triplicate on four separate occasions with data graphed being mean and error bars representing standard deviation.