Abstract
The immune system can be modulated and regulated not only by foreign antigens but also by other humoral factors and metabolic products, which are able to affect several quantitative and qualitative aspects of immunity. Among these, endocannabinoids are a group of bioactive lipids that might serve as secondary modulators, which when mobilized coincident with or shortly after first-line immune modulators, increase or decrease many immune functions. Most immune cells express these bioactive lipids, together with their set of receptors and of enzymes regulating their synthesis and degradation. In this review, a synopsis of the manifold immunomodulatory effects of endocannabinoids and their signalling in the different cell populations of innate and adaptive immunity is appointed, with a particular distinction between mice and human immune system compartments.
Keywords: cell signalling, endocannabinoids, immune cells
The endocannabinoid system
Although Δ9-tetrahydrocannabinol was isolated exactly 50 years ago, it was only at the beginning of the 1990s that cannabinoid receptors were described and cloned in the brain, so explaining why our body reacts to cannabis extracts and representing the first evidence for the presence of the so-called ‘endocannabinoid system’ (ECS). The discovery of cannabinoid receptors initiated a quest for their endogenous ligands, which progressively led to the identification and isolation of a new family of N- or O-derivatives of polyunsaturated fatty acids able to act as cannabinoid receptor agonists and collectively termed endocannabinoids (eCBs).1,2
The endocannabinoids and their metabolism
Endocannabinoids include a group of lipid mediators, of which the best characterized members are N-arachidonoylethanolamine (anandamide, AEA) and 2-arachidonoylglycerol (2-AG).3,4 Some other compounds have been proposed to belong to the eCB family, including 2-AG-ether (noladin ether) and O-arachidonoylethanolamine (virodhamine).2 Among these ‘eCB-like’ compounds, two additional N-acylethanolamines, namely N-palmitoylethanolamine (PEA) and N-oleoylethanolamine, have been extensively investigated because of their anti-inflammatory and analgesic properties,5–7 and anorexic effects, respectively.8 Endocannabinoids are synthesized and released ‘on demand’ (if and when needed) from membrane phospholipids in response to physiological or pathological stimuli. However, this ‘dogma’ has been lately reconsidered because of the discovery of intracellular transporters and storage organelles/pools that might serve as potential platforms for eCB trafficking and accumulation. This novel concept adds more complexity to eCB homeostasis and certainly makes them more available both for receptor activation and for distinct metabolic pathways, away from the site and time of their biosynthesis.9–12 Biosynthesis of AEA and of its congeners includes two steps: N-arachidonoyl-phosphatidylethanolamine is formed from phosphatidylethanolamine by calcium-dependent N-acyl-transferase, and is then converted through at least five distinct metabolic pathways into AEA or other N-acylethanolamines.13 The most studied route for such a conversion involves the N-acyl-phosphatidylethanolamine-hydrolysing phospholipase D,14 but other alternative yet relevant pathways engage phospholipase A and lyso-phospholipase D,15 α/β-hydrolase 4 and glycerophosphodiesterase 1,16 or phospholipase C and protein tyrosine phosphatase type-22.17 The biosynthesis of 2-AG starts from sn-1-acyl-2-arachidonoylglycerols (DAGs), that can be directly converted into 2-AG through the action of two Ca2+-sensitive sn-2-selective DAG lipases, i.e. DAGL-α and DAGL-β.18 A less-characterized pathway for 2-AG biosynthesis involves the generation of 2-AG-3-phosphate, which is a lysophosphatidic acid.19 All eCBs are then inactivated by a two-step process: cellular uptake through a purported ‘endocannabinoid membrane transporter’, whose molecular identity has yet to be identified, and intracellular hydrolysis. AEA is principally cleaved by fatty acid amide hydrolase (FAAH) into arachidonic acid and ethanolamine,20 but also by another enzyme, N-acylethanolamine-hydrolysing acid amidase, which is mainly involved in the hydrolysis of PEA and whose physiological implications are still unclear.13 2-AG can be cleaved into glycerol and arachidonic acid by FAAH, though its main hydrolase is a monoacylglycerol lipase, responsible for ∼ 85% of 2-AG hydrolysis in the mouse brain.21,22 In addition, 2-AG can also be cleaved by two integral membrane proteins, α/β-hydrolase domain-containing proteins 6 and 12.21,22 Furthermore, AEA and 2-AG are substrates of cyclooxygenase-2 (COX-2), different lipoxygenase isozymes and cytochrome P450, leading to oxidized compounds like prostaglandin-ethanolamides and -glyceryl esters, hydroxy-anandamides and hydroxyeicosatetraenoyl-glycerols, respectively, all endowed with distinct biological activities.22 The main elements of the ECS are schematically depicted in Fig.1.
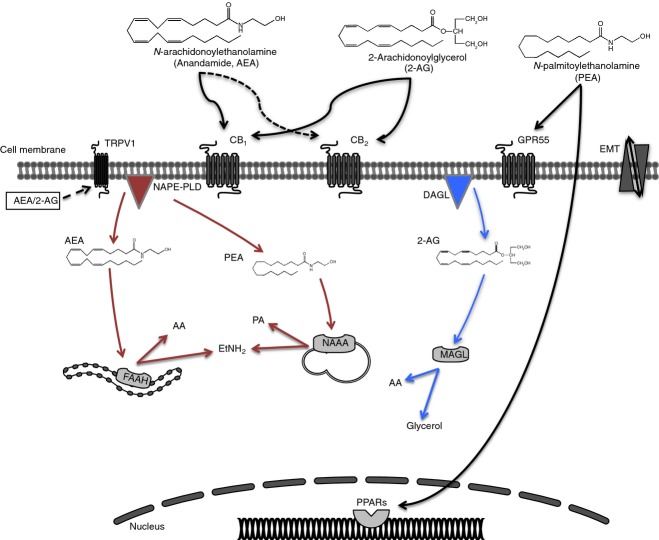
Figure 1.
Metabolism of the main immunoregulatory endocannabinoids (eCBs). N-Arachidonoylethanolamine (AEA) or N-palmitoylethanolamine (PEA) and 2-arachidonoylglycerol (2-AG) are usually released on demand from membrane lipids, through the activity of N-acyl-phosphatidylethanolamine-hydrolysing phospholipase D (NAPE-PLD) and sn-1-acyl-2-arachidonoylglycerol lipase (DAGL), respectively and move across the plasma membrane via a purported endocannabinoid membrane transporter (EMT). Targets of AEA and 2-AG are CB1 and CB2, which show an extracellular binding site. AEA also binds to transient receptor potential vanilloid 1 (TRPV1), which bears an intracellular binding site. PEA binds and activates peroxisome proliferator-activated receptors (PPARs) and G protein-coupled receptor 55 (GPR55). Dashed lines represent low-affinity bindings. Once eCBs bind to their target receptors, different signalling pathways can be activated depending on the cellular environment (see text for detail). After their actions, eCBs are taken up by EMT for inactivation; AEA is hydrolysed by fatty acid amide hydrolase (FAAH) to ethanolamine and arachidonic acid, 2-AG is hydrolysed by monoacylglycerol lipase (MAGL) and to a minor extent by FAAH, releasing glycerol and arachidonic acid and PEA is hydrolysed by N-acylethanolamine-hydrolysing acid amidase (NAAA) into ethanolamine and palmitic acid.
Molecular targets and signalling pathways
Once synthesized, eCBs bind to and functionally activate their target receptors, triggering various signalling pathways and causing several biological effects on different tissues (Fig.1). The main receptor targets for eCBs are type-1 (CB1) and type-2 (CB2) G protein-coupled cannabinoid receptors.23 CB1 is widely expressed in the nervous system, mainly at the terminal ends of central and peripheral neurons, and its presence has also been widely documented in many different extraneural sites. Once activated, CB1 is involved in the inhibition of excitatory and inhibitory neurotransmission and can modulate cognitive, memory and motor functions, as well as analgesia. CB2 is mainly expressed by cells of the immune system where it is commonly associated with the regulation of different immune functions.24 The identification of CB2 in brainstem neurons and its presence in activated microglial cells and astrocytes, or in certain subsets of neurons upon insult,25,26 has led to an ‘identity crisis’ of this receptor.27 Indeed, the up-regulation of CB2 is associated with chronic inflammation of the nervous system, as well as with several cardiovascular and bone disorders.28,29 CB1 and CB2 are metabotropic receptors that usually couple to heterotrimeric Gi alpha subunit proteins, and so trigger the canonical signalling pathway of inhibition of adenylyl cyclase activity and reduction of cAMP levels, which lead to the inactivation of protein kinase A. CB1 and CB2 also activate various effector protein kinase cascades involved in cell proliferation and survival; these include the phosphatidylinositol 3-kinase/protein kinase B, the mitogen-activated protein kinase p38, the extracellular-signalling regulated protein kinase-mitogen-activated protein kinase, as well as the focal adhesion kinase.29 Other signalling pathways include coupling to ion channels (N- and P/Q-type Ca2+ channels and voltage-gated K+ channels), activation of phospholipase-Cβ, and ceramide biosynthesis.29 In addition to CB1 and CB2, it is now clearly established that eCBs can engage other non-CB targets.23 The best known is the transient receptor potential vanilloid 1 channel, activated intracellularly by AEA and 2-AG,30,31 which is expressed in sensory neurons and in epithelial, endothelial and immune cells.32 Also peroxisome proliferator-activated receptor (PPAR) α and γ,33 which belong to a family of nuclear receptors able to alter lipid turnover and metabolism, as well as the orphan G protein-coupled receptor GPR55,34 are activated by eCBs. Probably these additional targets call for reconsideration of the name ‘cannabinoid receptor’, which might be readapted to take into consideration all the molecular targets identified so far for eCBs.
Role of ECS in the regulation of immune responses
Over the last 20 years, the ECS has been thoroughly studied in most cell types and tissues. Its role in the regulation of the immune system is probably the most flourishing and promising, mostly due to the increasing recognition of the eCBs signalling in several chronic inflammatory diseases. Also the fact that essentially all immune cells secrete eCBs, are capable of regulating their synthesis and degradation and possess cannabinoid receptors supports this view.35–38 It is now generally accepted that the immunosuppressive effects of eCBs on immune cells are primarily mediated through CB2, whose expression is usually higher than that of CB1.28,39 Unlike eCBs and their metabolizing enzymes, the presence and distribution of cannabinoid receptors within immune cells strongly vary and have been mainly investigated in human immune cell populations.40,41 Very few studies have addressed the differential expression of cannabinoid receptors on mouse immune cell subsets.42 Recently, a detailed analysis of CB2 protein levels expressed by the various blood immune cells from healthy human donors revealed that natural killer (NK) cells, B lymphocytes and monocytes express a higher level of CB2 than CD4+ or CD8+ T lymphocytes or neutrophils. However, NK cells have the greatest variation in CB2 expression levels, whereas for each of the other cell types CB2 levels are relatively similar between subjects.43 The low abundance of CB2 on resting T lymphocytes significantly increases on activated CD4+ and CD8+ human T cells. The current view is that eCBs, rather than just exerting either immunosuppressive or stimulatory effects on the immune system, are more likely to be part of a homeostatic immunoregulatory scheme. The majority of scientific studies on the immunoregulatory role of eCBs concentrated on whole immune cells, either on peripheral blood mononuclear cells or on mouse splenocytes, where AEA and PEA are mostly anti-inflammatory,44–46 and 2-AG exerts both pro-inflammatory and anti-inflammatory effects.47–50 Therefore, in this section we will address the immunoregulatory functions of the main eCBs and their signalling on the different immune cell populations of both innate and adaptive immunity, devoting special attention to whether they stem from peripheral human or murine immune cells, be they immortalized cell lines or primary cells. Indeed, mice are the most frequently used animal and the experimental tool of choice for the majority of immunologists. Study of their immune responses has yielded tremendous insight into the workings of the human immune system.51 However, a thorough demarcation is sought, not only because relevant differences exist between the immune system of humans and mice, but also because immortalized cell lines often respond differently from primary cells.
Endocannabinoid signalling in innate immunity
Monocytes/macrophages
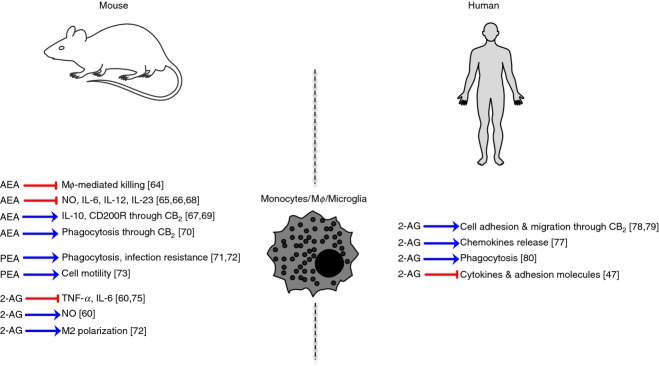
Macrophages (and their precursors, monocytes) play an important role in innate immunity, because they not only clear apoptotic cells and pathogens, but also instruct other immune cells. Monocytes/macrophages are highly plastic (they can change their functional phenotype depending on environmental cues) and reside in every tissue of the body, where they bear different names (i.e., Kupffer cells in the liver or microglia in the central nervous system).52 CB1 and CB2 receptors are highly expressed in both murine and human monocytes/macrophages and microglial cells, regardless of cellular models.41,42,53–57 Similarly, all eCB metabolic enzymes are often modulated in response to inflammatory stimuli, so regulating eCBs tone in vivo.58–61 Interestingly, a recent study reported the existence of bidirectional eCB transport across cell membranes of a monocytic cell line, combining both radioligand assays and quantification of intracellular and extracellular levels of AEA and 2-AG upon differential pharmacological blockage of their uptake, breakdown and interaction with binding proteins.62 These data extend a previous report on the in and out transport of AEA across human umbilical vein endothelial cells.63 The first evidence of an immunoregulatory role of eCBs on monocytes/macrophages came from a study on mouse alveolar macrophages, where AEA inhibited macrophage-mediated killing of tumour necrosis factor-sensitive cells.64 Later evidence supported the anti-inflammatory nature of AEA, according to which this endogenous lipid inhibited the expression of pro-inflammatory mediators such as nitric oxide and interleukins IL-6, IL-12 and IL-23, and enhanced anti-inflammatory mediators like IL-10 and CD200R. Nonetheless, these overt immunosuppressive effects were only seen in mouse macrophage cell lines and microglia60,65–69 and in most cases were mediated by CB2 signalling, whose involvement was also directly implicated in dectin-1-mediated phagocytosis.70 Also, PEA exerts anti-inflammatory properties on murine microglia, mainly by stimulating phagocytosis and clearance of pathogens, and by increasing resistance to infection and microglial cell motility.71–74 Conversely, there are scarce and contradictory data on the role of 2-AG in the modulation of mouse macrophage/microglia responses: on the one hand, 2-AG inhibits tumour necrosis factor-α (TNF-α) and IL-6 production and promotes alternatively activated and anti-inflammatory M2 macrophages;60,75,76 on the other hand, it increases inducible nitric oxide synthase-dependent nitric oxide production.60 Likewise, also in humans, 2-AG shows opposite effects. Indeed, it enhances the production of chemokines,76 migration and adhesion of macrophage-like differentiated human HL-60, U937 and THP-1 cell lines, as well as peripheral blood monocytes in a CB2-phosphatidylinositol 3-kinase-dependent pathway.77–79 Yet, 2-AG was also reported to enhance the phagocytosis of opsonized zymosan in the same cell lines,80 and to induce human monocytes to produce decreased levels of cytokines and adhesion molecules, thereby exhibiting an immunosuppressive response.47 In some cases, it was not entirely clear whether the effects of 2-AG were actually mediated via CB2 receptors. Incidentally, it has been suggested that discrepancies on the effects of 2-AG, and to a certain extent of AEA, could be due to their conversion into bioactive COX-2 metabolites.81 A summary of the main effects on mouse and human monocytes/macrophages is shown in Fig.2.
Figure 2.
Schematic representation of endocannabinoid (eCB) signalling in murine and human monocytes/macrophages or migroglia. Mϕ, macrophages.
Dendritic cells
Dendritic cells (DCs) are the most professional antigen-presenting cells, crucial in the development of antigen-specific T-cell responses. They are present in those tissues that are in contact with the external environment, such as the skin (i.e. Langerhans cells), and the inner lining of several organs; they can also be found in peripheral blood (i.e. myeloid and plasmacytoid DCs).82 Despite their role in shaping the type and quality of immune responses, due to their position at the crossroads between innate and adaptive immunity, very few studies have investigated endocannabinoid signalling in these cells, especially in humans (Fig.3). A pioneering study came from Di Marzo and coworkers, who demonstrated for the first time the presence of the ECS (AEA, 2-AG, PEA, CB1, CB2 and FAAH) in human blood monocyte-derived DCs, and its regulation upon cell activation. In particular, although the expression of CB1 and CB2 remained unmodified following cell maturation induced by lipopolysaccharide or by the allergen Der p I, the levels of 2-AG (but not those of AEA or PEA) were significantly increased.83 Following this first evidence, so far only one other study attempted to investigate the role of 2-AG in DCs, showing that it acts as a chemoattractant for both immature and mature bone marrow-derived mouse DCs. Additionally, 2-AG in vivo shifts the memory response towards the T helper type 1 (Th1) type.84 At the same time it was found that high (micromolar) doses of AEA induce apoptosis in murine bone marrow-derived DCs, through both CB1 and CB2 receptors, providing a potential mechanism for eCB-mediated immunosuppression of immune cells.85 Interestingly, the efficacy of AEA depended on its rapid hydrolysis by FAAH, because pharmacological inhibition of the latter led to a reduced resistance to apoptosis. The involvement of CB1 and CB2 in determining DC responses was clearly elucidated by analysing the phenotypic and functional profile of murine bone marrow-derived DCs from CB1–/– CB2–/– mice. Indeed, deletion of both cannabinoid receptors exacerbated DC function by increasing their activation markers (MHC-I/II, CD80, CD86) leading to a more mature phenotype, as well as by eliciting a more robust T-cell response.86 In contrast, it was reported that nanomolar and low micromolar doses of AEA before sensitization increased both the expression of murine DC co-stimulatory molecules (CD80/CD86) and IL-12/IL-23 production ex vivo.87 Yet, identification of these DC was somehow imprecise, because their immunophenotypic profile was carried out in total splenocytes stained only with CD11c, a marker shared also by other cell types. The only additional evidence on human DCs was obtained by our group on circulating peripheral blood myeloid and plasmacytoid DCs. Notably, we found that low micromolar doses of AEA significantly inhibited TNF-α, IL-12p40 and IL-6, as well as TNF-α and interferon-α, from activated myeloid and plasmacytoid DCs via CB2 respectively.88 Furthermore, such an AEA-mediated immunosuppression of both DC subsets was also paralleled by a reduced ability of myeloid and plasmacytoid DCs to polarize naive CD4 T cells into Th1 and Th17 lineages.88
Figure 3.
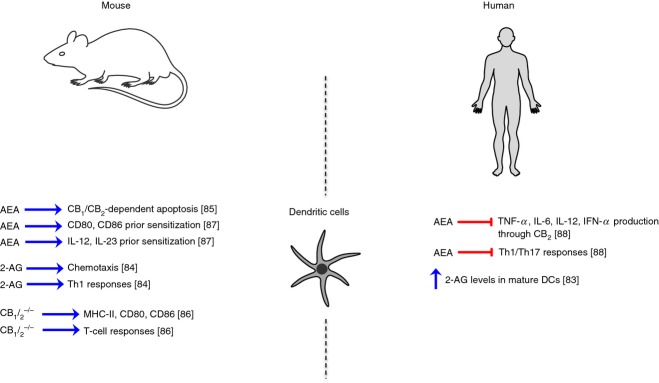
Schematic representation of endocannabinoid (eCB) signalling in murine and human dendritic cells.
Neutrophils and NK cells
Neutrophils and NK cells are crucial cells of innate immunity, and are both involved in host defence against cancer and anti-microbial responses. Neutrophils are the first inflammatory cells to be recruited at the site of inflammation/injury and are the hallmark of acute inflammation, whereas NK cells are a type of cytotoxic lymphocyte that provide rapid responses against virally infected cells and cancer cells.89,90 Surprisingly, although NK cells have been shown to express both CB1 and CB2 and to release high levels of AEA and 2-AG,54 knowledge of eCB signalling in NK cells is almost null and is summarized in Fig.4. Indeed, only two reports addressed the role of 2-AG in inducing the migration of the NK-differentiated human HL-60 cell line through CB2 receptor.91,92 This lack of evidence is probably a result of the difficulty in documenting cannabinoid receptors in NK cells, that show the greatest variation of expression of these receptors. However, our group has recently reported evidence that human peripheral blood NK cells express high levels of putative ‘CB3’ receptor, whose activation enhances NK cell functions in terms of TNF-α and interferon-γ production, and of CD107a-mediated cell killing.93 Instead, a great deal of information has been accumulated on the role of both AEA and 2-AG signalling on neutrophils in humans (Fig.4). Perhaps because of their abundance in peripheral blood, many studies have been performed on neutrophils. Although AEA has been shown to inhibit neutrophil migration94 and its levels positively correlated with their phagocytic capabilities,92 many studies consistently reported the failure of AEA to effectively inhibit superoxide and hydrogen peroxide production, so being almost inefficient in altering the microbicidal neutrophil burst reaction.95–97 At any rate, these effects seem to be independent of cannabinoid receptors. However, CB2 activation has been recently shown to reduce the release of metalloproteases from neutrophils, so potentially reducing vulnerability in atherosclerotic plaques.98 Conversely, 2-AG seems to be an activator of human neutrophils, by stimulating myeloperoxidase release, leukotriene B4 biosynthesis, kinase activation and calcium mobilization.99 It also induces increased levels of anti-microbial effectors, thereby being a potent regulator of host defence in vivo.100 As expected, these effects on neutrophil activation were not mediated by CB2, because of the very low levels of its expression in these cells, but were rather the result of its hydrolysis and subsequent metabolism into leukotriene B4, with activation of Leukotriene B4 receptors 1. Additional data supported a role for 2-AG in controlling RhoA activation, thereby suppressing neutrophil migration.101 Figure4 summarizes the main pathways of eCB signalling in neutrophils and NK cells.
Figure 4.
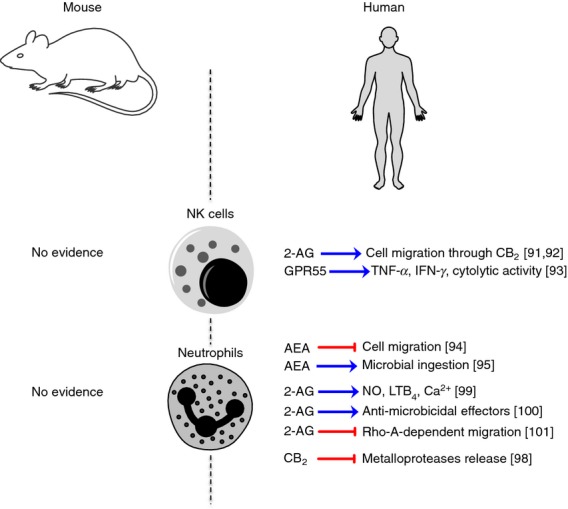
Schematic representation of endocannabinoid (eCB) signalling in murine and human natural killer cells and neutrophils.
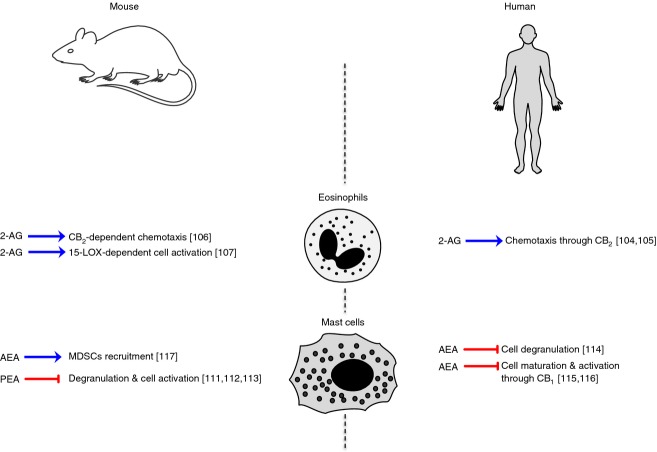
Eosinophils, basophils and mast cells
These rare cell populations share similar appearance and function and are involved in allergy and anaphylaxis as well as in wound healing and in defence against pathogens. However, they differ in that they arise from different cell lines and in that eosinophils and basophils are found in the blood whereas mast cells are tissue resident (i.e. connective and mucosal tissue, nervous system).102,103 Furthermore, eosinophils play a major role in dealing with elimination of large parasites.103 As yet, no evidence has been reported on eCB signalling for either murine or human basophils. Very few reports have addressed eosinophil response to eCBs, and to 2-AG in particular (Fig.5). The latter compound was found to induce the migration of human eosinophils in a CB2-dependent manner and consistently this receptor was particularly expressed in these cells.104 The same authors, by an ether-linked non-hydrolysable analogue of 2-AG, demonstrated that its migratory effect was attributable to chemotaxis and not to chemokinesis. Yet 2-AG potency was significantly lower than that of well-known and strong eosinophil chemoattractants, such as platelet-activating factor, RANTES and eotaxin.105 These studies suggest that CB2 and its endogenous ligand 2-AG may be potentially involved in allergic inflammation, accompanied by eosinophil infiltration, and this was indeed demonstrated in a mouse model of contact dermatitis.106 A recent paper investigated the mechanisms of 2-AG-induced migration of human eosinophils, confirming that this eCB in combination with IL-5 has the ability to activate and modulate eosinophil functional responses, and that the 15-lipoxygenase pathway is very probably involved in the regulation of these activities.107 Of note, the most studied eCB in allergy is PEA. Indeed, this substance has been extensively investigated in mast cells (especially in wild-type rats) that produce high levels of PEA and express both CB1 and CB2.108–110 On murine mast cells, PEA is a strong inhibitor of mast cell degranulation and activation,111 also contributing to reduce the severity of spinal cord trauma.112 Interestingly, a recent work hypothesized that the anti-nociceptive role of PEA in inducing relief in neuropathic pain correlates with its modulation of these non-neuronal cells.113 Also AEA has been shown to inhibit mast cell degranulation in a human mast cell line, where this lipid was effectively degraded through a nitric oxide-sensitive endocannabinoid membrane transporter and FAAH.114 This was confirmed 10 years later, demonstrating that AEA limits excessive mast cell maturation and activation in a CB1-dependent mechanism in a human hair follicle organ culture model, suggesting that normal skin mast cells are indeed modulated by the ECS.115 The involvement of AEA and CB1 in modulating human mast cell functions was further confirmed by the observation that in human airway mucosal mast cells, maturation and excessive activation were inhibited by the endocannabinoid tone through CB1 stimulation.116 A very recent and interesting work further unravelled the biological implication of AEA-CB1-mediated mast cell modulation in mast cell-deficient mice, showing that AEA activation of CB1 in mast cells induced monocyte chemotactic protein-1-mediated recruitment of monocytic and anti-inflammatory myeloid-derived suppressor cells.117 The main effects of PEA and AEA on murine and human mast cells are summarized in Fig.5.
Figure 5.
Schematic representation of endocannabinoid (eCB) signalling in murine and human eosinophils and mast cells.
Endocannabinoid signalling in adaptive immunity
T lymphocytes
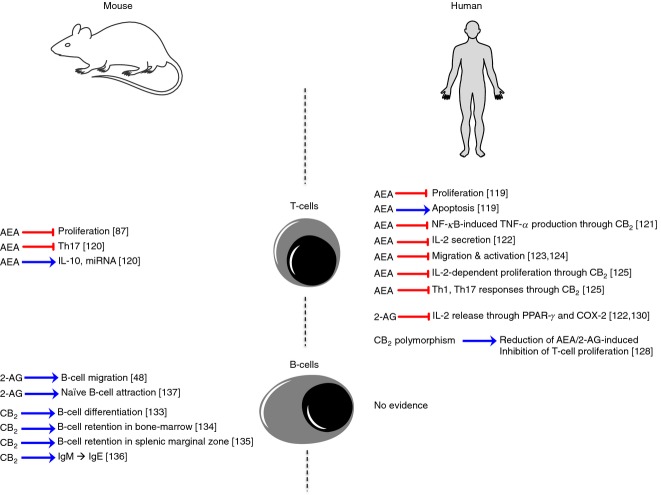
T lymphocytes (or T cells) play a central role in cell-mediated immunity, and comprise several subsets, each with a distinct function, including CD4+ T helper cells, CD8+ cytotoxic T cells, memory T cells, regulatory T cells and mucosal-associated invariant T cells.118 The first evidence for an immunosuppressive role of eCBs on T cells came as early as 2 years after the isolation and purification of AEA, demonstrating its dose-dependent anti-proliferative effects on human T cells. Indeed, micromolar doses of AEA rapidly inhibited mitogen-induced DNA synthesis, and this was associated with induction of apoptotic cell death.119 Since then, interest was primarily focused on phytocannabinoids and synthetic agonists/antagonists selective for CB1 or CB2. Only after more than 10 years did study of the immunomodulatory properties of eCBs on T cells begin to flourish – especially for AEA, which is the most studied eCB compared with 2-AG or PEA, which received little investigation. It is now accepted that AEA is a potent immunosuppressor of T-cell proliferation and cytokine release, acting mainly through CB2 and PPAR-γ and most likely through nuclear factor-κB inhibition. This pathway has been largely investigated in mouse T cells,87,120 in the human Jurkat T-cell line121,122 and in human peripheral T lymphocytes.123,124 Our group was the first to demonstrate the anti-proliferative effect of AEA on both CD4 and CD8 T-cell subsets, without any effect on cell viability.125 In addition, we disclosed its inhibitory effect on interferon-γ-producing Th1 and IL-17-producing Th17. This effect of AEA on Th17 has been recently reproduced in a mouse model of hypersensitivity, where it was also shown to be mediated by IL-10 and mitochondrial RNA induction.120 Interestingly, cytokines have been shown to directly influence the ECS of T lymphocytes, inasmuch as the Th2 cytokines IL-4 or IL-10 had a stimulatory effect on FAAH, whereas the Th1 cytokines IL-12 and interferon-γ reduced FAAH activity and protein expression,126 overall suggesting an eCB-triggered self-sustaining anti-inflammatory loop. In disagreement with these results, Lissoni et al. reported that AEA does not inhibit human T-cell proliferation and cytokine production, probably because of the presence of albumin in their in vitro experiments, which is known to bind AEA and so reduce its biological activity.127 The strong involvement of CB2 in mediating AEA anti-inflammatory effects is supported by a reduction of eCB immune modulation of T cells from a common CB2 polymorphism,128 and the evidence that formation of T cells requires this receptor.129 The anti-inflammatory role of 2-AG on T cells, instead, was shown to be independent of cannabinoid receptors, and its significant suppression of IL-2 expression in Jurkat T cells was mediated by a COX-2 metabolite of 2-AG, probably by activating the PPAR-γ.122,130
B lymphocytes
B lymphocytes (or B cells) are involved in the production of antibodies against antigens (humoral immunity), but they are also capable of acting as antigen-presenting cells.131 Antibody-producing plasma cells are among the immune cells that express the highest levels of CB2, with human B cells expressing one transcript and mouse B cells expressing three transcripts, specifically selected during B-cell activation by lipopolysaccharide.132 However, most of the research has focused only on the use of phytocannabinoids and ‘syntho-cannabinoids’, rather than on eCBs, trying to understand the functional role of this receptor in B cells. Indeed, CB2 was identified as a crucial receptor for mouse B-cell differentiation at the end of the 1990s, as it was markedly expressed in mantle zones of secondary follicles and less in germinal centres, and its expression was down-regulated during B-cell differentiation.133 Furthermore, CB2 was found to be essential also for mouse B-cell subset formation,129 and for retention of immature B cells in bone marrow sinusoids134 and in splenic marginal zones.135 CB2 was also reported to mediate immunoglobulin class switching from IgM to IgE,136 suggesting that this cannabinoid receptor could have a crucial role in the generation of B-cell repertoire and the regulation of Th2-type humoral responses. Only two works investigated the role of eCBs, in particular of 2-AG, in mouse B-cell functions, showing that this bioactive lipid induced migration of B220+ CD19+ B cells,48 preferentially by attracting unstimulated naive B cells rather than activated and/or class-switched germinal centre B cells in a CB2-dependent manner.137 Surprisingly, no evidence of eCB signalling on human B cells has been gathered. Furthermore, it is yet to be explored whether the effects on mouse B cells are direct or are indirectly induced through other immune cells (like T cells and macrophages), required for B-cell activation. Figure6 summarizes the principal effects on both T and B lymphocytes.
Figure 6.
Schematic representation of endocannabinoid (eCB) signalling in murine and human T and B lymphocytes.
Concluding remarks
Research on the ECS on the immune system strongly suggests that its lipid mediators and their receptors exert pleiotropic and complex immunoregulatory effects. The generally immunosuppressive role of classical eCBs (AEA and 2-AG) on the different immune cell populations is abundant compared with their congeners (PEA) and is reasonably equivalent in both mice and humans, making them ‘master regulators’ of the innate-adaptive immune axis. Although some immune cells can respond to the different eCBs, it seems that their effects are strictly dependent on cell type; for instance, T cells mainly respond to AEA, whereas eosinophils respond to 2-AG. Moreover, not only have the effects of eCBs been reported for some immune cells (NK cells, neutrophils and B cells) on mouse or human cell populations, but also some immune cells (regulatory T cells, γδ T cells, or mucosal-associated invariant T cells) have never been investigated and neither have the subpopulations of each innate or immune cell type. Although the immunomodulatory effects of eCBs mainly result from either in vitro or ex vivo studies, their corresponding functions in vivo require further confirmation and need to be fully elucidated, along with their underlying molecular mechanisms. Although these studies support the proposition that the CB2 receptor may represent a novel pharmacological target for selective agonists designed to suppress autoreactive immune responses while avoiding CB1 receptor-dependent psychoactive adverse effects, it seems that the modulation of the endocannabinoid levels by specifically inhibiting their breakdown enzymes (such as FAAH) or by inducing their production can provide a new avenue of research in the regulation of immune responses. On this basis and also considering the similar effects of eCBs in both mice and humans, autoimmune models of disease represent a valuable setting in which to study the pharmacological modulation of the ECS, especially in the light of the fact that the vast majority of immunomodulatory/immunosuppressant drugs available to clinicians for the treatment of several autoimmune diseases carry as a side effect the occurrence of infective diseases. In this context, as far as the eCB signalling is concerned, the risk of overlooking aspects of human immunology that cannot be modelled in mice, so precluding a translation into human clinical trials, seems to be minimal, yet calls for caution. Hence improvements in studies of the pathophysiological functions of eCB signalling and its modulation will help to translate this knowledge into the clinical setting to develop new immunomodulatory therapies or refine existing ones.
Acknowledgments
We wish to thank Professor Giorgio Bernardi for his continuing support of our studies on immunoregulation of endocannabinoid signalling and Dr Giovanna Borsellino for helpful discussion and her precious contribution in the final editing of the manuscript. Financial support from Ministero dell’Istruzione, dell’Università e della Ricerca (PRIN 2011 grant) to MM, from the Ministero della Salute (RF-2011-02346771) to LB and from Fondazione Italiana Sclerosi Multipla (FISM grant 2013/R/8) to VC and (FISM grant 2013/R/2) to LB is gratefully acknowledged.
Disclosures
The authors declare no competing interests.
References
- Di Marzo V, Fontana A. Anandamide, an endogenous cannabinomimetic eicosanoid:’killing two birds with one stone’. Prostaglandins Leukot Essent Fatty Acids. 1995;53:1–11. doi: 10.1016/0952-3278(95)90077-2. [DOI] [PubMed] [Google Scholar]
- Fezza F, Bari M, Florio R, Talamonti E, Feole M, Maccarrone M. Endocannabinoids, related compounds and their metabolic routes. Molecules. 2014;19:17078–106. doi: 10.3390/molecules191117078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane WA, Hannus L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–9. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Thabuis C, Tissot-Favre D, Bezelgues JB, Martin JC, Cruz-Hernandez C, Dionisi F, Destaillats F. Biological functions and metabolism of oleoylethanolamide. Lipids. 2008;43:887–94. doi: 10.1007/s11745-008-3217-y. [DOI] [PubMed] [Google Scholar]
- Di Cesare Mannelli L, D’Agostino G, Pacini A, Russo R, Zanardelli M, Ghelardini C, Calignano A. Palmitoylethanolamide is a disease-modifying agent in peripheral neuropathy: pain relief and neuroprotection share a PPAR-α-mediated mechanism. Mediators Inflamm. 2013;2013:328797. doi: 10.1155/2013/328797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen HS. Palmitoylethanolamide and other anandamide congeners. Proposed role in the diseased brain. Exp Neurol. 2010;224:48–55. doi: 10.1016/j.expneurol.2010.03.022. [DOI] [PubMed] [Google Scholar]
- Gaetani S, Kaye WH, Cuomo V, Piomelli D. Role of endocannabinoids and their analogues in obesity and eating disorders. Eat Weight Disord. 2008;13:e42–6. [PubMed] [Google Scholar]
- Oddi S, Fezza F, Pasquariello N, De Simone C, Rapino C, Dainese E, Finazzi-Agrò A, Maccarrone M. Evidence for the intracellular accumulation of anandamide in adiposomes. Cell Mol Life Sci. 2008;65:840–50. doi: 10.1007/s00018-008-7494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddi S, Fezza F, Pasquariello N, et al. Molecular identification of albumin and Hsp70 as cytosolic anandamide-binding proteins. Chem Biol. 2009;16:624–32. doi: 10.1016/j.chembiol.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Kaczocha M, Glaser ST, Chae J, Brown DA, Deutsch DG. Lipid droplets are novel sites of N-acylethanolamine inactivation by fatty acid amide hydrolase-2. J Biol Chem. 2010;285:2796–806. doi: 10.1074/jbc.M109.058461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M, Dainese E, Oddi S. Intracellular trafficking of anandamide: new concepts for signaling. Trends Biochem Sci. 2010;35:601–8. doi: 10.1016/j.tibs.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Ueda N, Tsuboi K, Uyama T. Metabolism of endocannabinoids and related N-acylethanolamines: canonical and alternative pathways. FEBS J. 2013;9:1874–94. doi: 10.1111/febs.12152. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem. 2004;279:5298–305. doi: 10.1074/jbc.M306642200. [DOI] [PubMed] [Google Scholar]
- Sun YX, Tsuboi K, Okamoto Y, Tonai T, Murakami M, Kudo I, Ueda N. Biosynthesis of anandamide and N-palmitoylethanolamine by sequential actions of phospholipase A2 and lysophospholipase D. Biochem J. 2004;380:749–56. doi: 10.1042/BJ20040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon GM, Cravatt BF. Characterization of mice lacking candidate N-acyl ethanolamine biosynthetic enzymes provides evidence for multiple pathways that contribute to endocannabinoid production in vivo. Mol BioSyst. 2010;6:1411–8. doi: 10.1039/c000237b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang L, Harvey-White J, et al. A biosynthetic pathway for anandamide. Proc Natl Acad Sci USA. 2006;103:13345–50. doi: 10.1073/pnas.0601832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Howell F, Williams G, et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–8. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane S, Oka S, Arai S, Waku K, Ishima Y, Tokumura A, Sugiura T. 2-Arachidonoyl-sn-glycero-3-phosphate, an arachidonic acid-containing lysophosphatidic acid: occurrence and rapid enzymatic conversion to 2-arachidonoyl-sn-glycerol, a cannabinoid receptor ligand, in rat brain. Arch Biochem Biophys. 2002;402:51–8. doi: 10.1016/S0003-9861(02)00038-3. [DOI] [PubMed] [Google Scholar]
- McKinney MK, Cravatt BF. Structure and function of fatty acid amide hydrolase. Annu Rev Biochem. 2005;74:411–32. doi: 10.1146/annurev.biochem.74.082803.133450. [DOI] [PubMed] [Google Scholar]
- Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–56. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs WR, Blankman JL, Horne EA, et al. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat Neurosci. 2010;13:951–7. doi: 10.1038/nn.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, Howlett AC, Abood ME, et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. 2010;62:588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Dittel BN. Unraveling the complexities of cannabinoid receptor 2 (CB2) immune regulation in health and disease. Immunol Res. 2011;51:26–38. doi: 10.1007/s12026-011-8210-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–32. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, et al. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann N Y Acad Sci. 2006;1074:514–36. doi: 10.1196/annals.1369.052. [DOI] [PubMed] [Google Scholar]
- Atwood BK, Mackie K. CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol. 2010;3:467–79. doi: 10.1111/j.1476-5381.2010.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel KD, Davison JS, Pittman QJ, Sharkey KA. Cannabinoid CB2 receptors in health and disease. Curr Med Chem. 2010;17:1393–410. doi: 10.2174/092986710790980041. [DOI] [PubMed] [Google Scholar]
- Galve-Roperh I, Chiurchiù V, Díaz-Alonso J, Bari M, Guzmán M, Maccarrone M. Cannabinoid receptor signaling in progenitor/stem cell proliferation and differentiation. Prog Lipid Res. 2013;52:633–50. doi: 10.1016/j.plipres.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L. Endocannabinoids as regulators of transient receptor potential (TRP) channels: a further opportunity to develop new endocannabinoid-based therapeutic drugs. Curr Med Chem. 2010;17:1430–49. doi: 10.2174/092986710790980078. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Ermund A, Movahed P, et al. Monoacylglycerols activate TRPV1 – a link between phospholipase C and TRPV1. PLoS ONE. 2013;8:e81618. doi: 10.1371/journal.pone.0081618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia R, Samad TA, Btesh J, Jiang LH, Kays I, Stjernborg L, Dekker N. TRPV1 signaling: mechanistic understanding and therapeutic potential. Curr Top Med Chem. 2011;11:2180–91. doi: 10.2174/156802611796904843. [DOI] [PubMed] [Google Scholar]
- Pistis M, Melis M. From surface to nuclear receptors: the endocannabinoid family extends its assets. Curr Med Chem. 2010;17:1450–67. doi: 10.2174/092986710790980014. [DOI] [PubMed] [Google Scholar]
- Moriconi A, Cerbara I, Maccarrone M, Topai A. GPR55: current knowledge and future perspectives of a purported “Type-3” cannabinoid receptor. Curr Med Chem. 2010;17:1411–29. doi: 10.2174/092986710790980069. [DOI] [PubMed] [Google Scholar]
- Centonze D, Battistini L, Maccarrone M. The endocannabinoid system in peripheral lymphocytes as a mirror of neuroinflammatory diseases. Curr Pharm Des. 2008;14:2370–82. doi: 10.2174/138161208785740018. [DOI] [PubMed] [Google Scholar]
- Pandey R, Mousawy K, Nagarkatti M, Nagarkatti P. Endocannabinoids and immune regulation. Pharmacol Res. 2009;60:85–92. doi: 10.1016/j.phrs.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanasescu R, Gran B, Constantinescu CS. The endocannabinoid system: a revolving plate in neuro-immune interaction in health and disease. Amino Acids. 2013;45:95–112. doi: 10.1007/s00726-012-1252-8. [DOI] [PubMed] [Google Scholar]
- Sánchez López AJ, Román-Vega L, Ramil Tojeiro E, Giuffrida A, García-Merino A. Regulation of cannabinoid receptor gene expression and endocannabinoid levels in lymphocyte subsets by IFN-β: a longitudinal study in multiple sclerosis patients. Clin Exp Immunol. 2014;179:119–27. doi: 10.1111/cei.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister SD, Glass M. CB1 and CB2 receptor-mediated signalling: a focus on endocannabinoids. Prostaglandins Leukot Essent Fatty Acids. 2002;66:161–71. doi: 10.1054/plef.2001.0344. [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Rinaldi M, Carayon P, Carillon C, Delpech B, Shire D, Le Fur G, Casellas P. Cannabinoid-receptor expression in human leukocytes. Eur J Biochem. 1993;214:173–80. doi: 10.1111/j.1432-1033.1993.tb17910.x. [DOI] [PubMed] [Google Scholar]
- Galiègue S, Mary S, Marchand J, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Lee SF, Newton C, Widen R, Friedman H, Klein TW. Differential expression of cannabinoid CB2 receptor mRNA in mouse immune cell subpopulations and following B cell stimulation. Eur J Pharmacol. 2001;423:235–41. doi: 10.1016/s0014-2999(01)01122-0. [DOI] [PubMed] [Google Scholar]
- Graham ES, Angel CE, Schwarcz LE, Dunbar PR, Glass M. Detailed characterisation of CB2 receptor protein expression in peripheral blood immune cells from healthy human volunteers using flow cytometry. Int J Immunopathol Pharmacol. 2010;23:25–34. doi: 10.1177/039463201002300103. [DOI] [PubMed] [Google Scholar]
- Berdyshev EV, Boichot E, Germain N, Allain N, Anger JP, Lagente V. Influence of fatty acid ethanolamides and Δ9-tetrahydrocannabinol on cytokine and arachidonate release by mononuclear cells. Eur J Pharmacol. 1997;330:231–40. doi: 10.1016/s0014-2999(97)01007-8. [DOI] [PubMed] [Google Scholar]
- Eisenstein TK, Meissler JJ, Wilson Q, Gaughan JP, Adler MW. Anandamide and Δ9-tetrahydrocannabinol directly inhibit cells of the immune system via CB2 receptors. J Neuroimmunol. 2007;189:17–22. doi: 10.1016/j.jneuroim.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solorzano C, Zhu C, Battista N, et al. Selective N-acylethanolamine-hydrolyzing acid amidase inhibition reveals a key role for endogenous palmitoylethanolamide in inflammation. Proc Natl Acad Sci U S A. 2009;106:20966–71. doi: 10.1073/pnas.0907417106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefano GB, Bilfinger TV, Rialas CM, Deutsch DG. 2-Arachidonyl-glycerol stimulates nitric oxide release from human immune and vascular tissues and invertebrate immunocytes by cannabinoid receptor 1. Pharmacol Res. 2000;42:317–22. doi: 10.1006/phrs.2000.0702. [DOI] [PubMed] [Google Scholar]
- Jordà MA, Verbakel SE, Valk PJ, Vankan-Berkhoudt YV, Maccarrone M, Finazzi-Agrò A, Löwenberg B, Delwel R. Hematopoietic cells expressing the peripheral cannabinoid receptor migrate in response to the endocannabinoid 2-arachidonoylglycerol. Blood. 2002;99:2786–93. doi: 10.1182/blood.v99.8.2786. [DOI] [PubMed] [Google Scholar]
- Ouyang Y, Hwang SG, Han SH, Kaminski NE. Suppression of interleukin-2 by the putative endogenous cannabinoid 2-arachidonyl-glycerol is mediated through down-regulation of the nuclear factor of activated T cells. Mol Pharmacol. 1998;53:676–83. doi: 10.1124/mol.53.4.676. [DOI] [PubMed] [Google Scholar]
- Kaplan BL, Ouyang Y, Rockwell CE, Rao GK, Kaminski NE. 2-Arachidonoyl-glycerol suppresses interferon-γ production in phorbol ester/ionomycin-activated mouse splenocytes independent of CB1 or CB2. J Leukoc Biol. 2005;77:966–74. doi: 10.1189/jlb.1104652. [DOI] [PubMed] [Google Scholar]
- Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–8. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014;14:392–404. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- Sun YX, Tsuboi K, Zhao LY, Okamoto Y, Lambert DM, Ueda N. Involvement of N-acylethanolamine-hydrolyzing acid amidase in the degradation of anandamide and other N-acylethanolamines in macrophages. Biochim Biophys Acta. 2005;1736:211–20. doi: 10.1016/j.bbalip.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Maresz K, Carrier EJ, Ponomarev ED, Hillard CJ, Dittel BN. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J Neurochem. 2005;95:437–45. doi: 10.1111/j.1471-4159.2005.03380.x. [DOI] [PubMed] [Google Scholar]
- Jiang LS, Pu J, Han ZH, Hu LH, He B. Role of activated endocannabinoid system in regulation of cellular cholesterol metabolism in macrophages. Cardiovasc Res. 2009;81:805–13. doi: 10.1093/cvr/cvn344. [DOI] [PubMed] [Google Scholar]
- Sugamura K, Sugiyama S, Nozaki T, et al. Activated endocannabinoid system in coronary artery disease and antiinflammatory effects of cannabinoid 1 receptor blockade on macrophages. Circulation. 2009;119:28–36. doi: 10.1161/CIRCULATIONAHA.108.811992. [DOI] [PubMed] [Google Scholar]
- Chiurchiù V, Lanuti M, Catanzaro G, Fezza F, Rapino C, Maccarrone M. Detailed characterization of the endocannabinoid system in human macrophages and foam cells, and anti-inflammatory role of type-2 cannabinoid receptor. Atherosclerosis. 2014;233:55–63. doi: 10.1016/j.atherosclerosis.2013.12.042. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L, Sepe N, Buono A. Biosynthesis of anandamide and related acylethanolamides in mouse J774 macrophages and N18 neuroblastoma cells. Biochem J. 1996;316:977–84. doi: 10.1042/bj3160977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Maurelli S, Melck D, De Petrocellis L, Di Marzo V. Biosynthesis, uptake, and degradation of anandamide and palmitoylethanolamide in leukocytes. J Biol Chem. 1997;272:3315–23. doi: 10.1074/jbc.272.6.3315. [DOI] [PubMed] [Google Scholar]
- Chang YH, Lee ST, Lin WW. Effects of cannabinoids on LPS-stimulated inflammatory mediator release from macrophages: involvement of eicosanoids. J Cell Biochem. 2001;81:715–23. doi: 10.1002/jcb.1103. [DOI] [PubMed] [Google Scholar]
- Zhu C, Solorzano C, Sahar S, Realini N, Fung E, Sassone-Corsi P, Piomelli D. Proinflammatory stimuli control N-acylphosphatidylethanolamine-specific phospholipase D expression in macrophages. Mol Pharmacol. 2011;79:786–92. doi: 10.1124/mol.110.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicca A, Marazzi J, Nicolussi S, Gertsch J. Evidence for bidirectional endocannabinoid transport across cell membranes. J Biol Chem. 2012;287:34660–82. doi: 10.1074/jbc.M112.373241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M, Bari M, Battista N, Finazzi-Agrò A. Estrogen stimulates arachidonoylethanolamide release from human endothelial cells and platelet activation. Blood. 2002;100:4040–8. doi: 10.1182/blood-2002-05-1444. [DOI] [PubMed] [Google Scholar]
- Cabral GA, Toney DM, Fischer-Stenger K, Harrison MP, Marciano-Cabral F. Anandamide inhibits macrophage-mediated killing of tumor necrosis factor-sensitive cells. Life Sci. 1995;56:2065–72. doi: 10.1016/0024-3205(95)00190-h. [DOI] [PubMed] [Google Scholar]
- Ross RA, Brockie HC, Pertwee RG. Inhibition of nitric oxide production in RAW264.7 macrophages by cannabinoids and palmitoylethanolamide. Eur J Pharmacol. 2000;401:121–30. doi: 10.1016/s0014-2999(00)00437-4. [DOI] [PubMed] [Google Scholar]
- Correa F, Docagne F, Clemente D, Mestre L, Becker C, Guaza C. Anandamide inhibits IL-12p40 production by acting on the promoter repressor element GA-12: possible involvement of the COX-2 metabolite prostamide E2. Biochem J. 2008;409:761–70. doi: 10.1042/BJ20071329. [DOI] [PubMed] [Google Scholar]
- Correa F, Hernangómez M, Mestre L, Loría F, Spagnolo A, Docagne F, Di Marzo V, Guaza C. Anandamide enhances IL-10 production in activated microglia by targeting CB2 receptors: roles of ERK1/2, JNK, and NF-κB. Glia. 2010;58:135–47. doi: 10.1002/glia.20907. [DOI] [PubMed] [Google Scholar]
- Correa F, Hernangómez-Herrero M, Mestre L, Loría F, Docagne F, Guaza C. The endocannabinoid anandamide downregulates IL-23 and IL-12 subunits in a viral model of multiple sclerosis: evidence for a cross-talk between IL-12p70/IL-23 axis and IL-10 in microglial cells. Brain Behav Immun. 2011;25:736–49. doi: 10.1016/j.bbi.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Hernangómez M, Mestre L, Correa FG, et al. CD200-CD200R1 interaction contributes to neuroprotective effects of anandamide on experimentally induced inflammation. Glia. 2012;60:1437–50. doi: 10.1002/glia.22366. [DOI] [PubMed] [Google Scholar]
- Shiratsuchi A, Watanabe I, Yoshida H, Nakanishi Y. Involvement of cannabinoid receptor CB2 in dectin-1-mediated macrophage phagocytosis. Immunol Cell Biol. 2008;86:179–84. doi: 10.1038/sj.icb.7100121. [DOI] [PubMed] [Google Scholar]
- Redlich S, Ribes S, Schütze S, Czesnik D, Nau R. Palmitoylethanolamide stimulates phagocytosis of Escherichia coli K1 and Streptococcus pneumoniae R6 by microglial cells. J Neuroimmunol. 2012;244:32–4. doi: 10.1016/j.jneuroim.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Redlich S, Ribes S, Schütze S, Nau R. Palmitoylethanolamide stimulates phagocytosis of Escherichia coli K1 by macrophages and increases the resistance of mice against infections. J Neuroinflammation. 2014;11:108. doi: 10.1186/1742-2094-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin A, Parmentier-Batteur S, Walter L, Greenberg DA, Stella N. Palmitoylethanolamide increases after focal cerebral ischemia and potentiates microglial cell motility. J Neurosci. 2003;23:7767–75. doi: 10.1523/JNEUROSCI.23-21-07767.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaper SD, Facci L, Giusti P. Mast cells, glia and neuroinflammation: partners in crime? Immunology. 2014;141:314–27. doi: 10.1111/imm.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallily R, Breuer A, Mechoulam R. 2-Arachidonylglycerol, an endogenous cannabinoid, inhibits tumor necrosis factor-α production in murine macrophages, and in mice. Eur J Pharmacol. 2000;406:R5–7. doi: 10.1016/s0014-2999(00)00653-1. [DOI] [PubMed] [Google Scholar]
- Lourbopoulos A, Grigoriadis N, Lagoudaki R, et al. Administration of 2-arachidonoylglycerol ameliorates both acute and chronic experimental autoimmune encephalomyelitis. Brain Res. 2011;1390:126–41. doi: 10.1016/j.brainres.2011.03.020. [DOI] [PubMed] [Google Scholar]
- Kishimoto S, Kobayashi Y, Oka S, Gokoh M, Waku K, Sugiura T. 2-Arachidonoylglycerol, an endogenous cannabinoid receptor ligand, induces accelerated production of chemokines in HL-60 cells. J Biochem. 2004;135:517–24. doi: 10.1093/jb/mvh063. [DOI] [PubMed] [Google Scholar]
- Kishimoto S, Gokoh M, Oka S, Muramatsu M, Kajiwara T, Waku K, Sugiura T. 2-Arachidonoylglycerol induces the migration of HL-60 cells differentiated into macrophage-like cells and human peripheral blood monocytes through the cannabinoid CB2 receptor-dependent mechanism. J Biol Chem. 2003;278:24469–75. doi: 10.1074/jbc.M301359200. [DOI] [PubMed] [Google Scholar]
- Gokoh M, Kishimoto S, Oka S, Metani Y, Sugiura T. 2-Arachidonoylglycerol, an endogenous cannabinoid receptor ligand, enhances the adhesion of HL-60 cells differentiated into macrophage-like cells and human peripheral blood monocytes. FEBS Lett. 2005;579:6473–8. doi: 10.1016/j.febslet.2005.10.030. [DOI] [PubMed] [Google Scholar]
- Gokoh M, Kishimoto S, Oka S, Sugiura T. 2-Arachidonoylglycerol enhances the phagocytosis of opsonized zymosan by HL-60 cells differentiated into macrophage-like cells. Biol Pharm Bull. 2007;30:1199–205. doi: 10.1248/bpb.30.1199. [DOI] [PubMed] [Google Scholar]
- Alhouayek M, Muccioli GG. COX-2-derived endocannabinoid metabolites as novel inflammatory mediators. Trends Pharmacol Sci. 2014;35:284–92. doi: 10.1016/j.tips.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias I, Pochard P, Orlando P, Salzet M, Pestel J, Di Marzo V. Presence and regulation of the endocannabinoid system in human dendritic cells. Eur J Biochem. 2002;269:3771–8. doi: 10.1046/j.1432-1033.2002.03078.x. [DOI] [PubMed] [Google Scholar]
- Maestroni GJ. The endogenous cannabinoid 2-arachidonoyl glycerol as in vivo chemoattractant for dendritic cells and adjuvant for Th1 response to a soluble protein. FASEB J. 2004;18:1914–6. doi: 10.1096/fj.04-2190fje. [DOI] [PubMed] [Google Scholar]
- Do Y, McKallip RJ, Nagarkatti M, Nagarkatti PS. Activation through cannabinoid receptors 1 and 2 on dendritic cells triggers NF-κB-dependent apoptosis: novel role for endogenous and exogenous cannabinoids in immunoregulation. J Immunol. 2004;173:2373–82. doi: 10.4049/jimmunol.173.4.2373. [DOI] [PubMed] [Google Scholar]
- Karmaus PW, Chen W, Crawford RB, Harkema JR, Kaplan BL, Kaminski NE. Deletion of cannabinoid receptors 1 and 2 exacerbates APC function to increase inflammation and cellular immunity during influenza infection. J Leukoc Biol. 2011;90:983–95. doi: 10.1189/jlb.0511219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro A, Ferraz-de-Paula V, Pinheiro ML, Sakai M, Costa-Pinto FA, Palermo-Neto J. Anandamide prior to sensitization increases cell-mediated immunity in mice. Int Immunopharmacol. 2010;10:431–9. doi: 10.1016/j.intimp.2009.12.017. [DOI] [PubMed] [Google Scholar]
- Chiurchiù V, Cencioni MT, Bisicchia E, et al. Distinct modulation of human myeloid and plasmacytoid dendritic cells by anandamide in multiple sclerosis. Ann Neurol. 2013;73:626–36. doi: 10.1002/ana.23875. [DOI] [PubMed] [Google Scholar]
- Mócsai A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med. 2013;210:1283–99. doi: 10.1084/jem.20122220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–9. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Oka S, Gokoh M, Kishimoto S, Waku K. New perspectives in the studies on endocannabinoid and cannabis: 2-arachidonoylglycerol as a possible novel mediator of inflammation. J Pharmacol Sci. 2004;96:367–75. doi: 10.1254/jphs.fmj04003x3. [DOI] [PubMed] [Google Scholar]
- Kishimoto S, Muramatsu M, Gokoh M, Oka S, Waku K, Sugiura T. Endogenous cannabinoid receptor ligand induces the migration of human natural killer cells. J Biochem. 2005;137:217–23. doi: 10.1093/jb/mvi021. [DOI] [PubMed] [Google Scholar]
- Chiurchiù V, Lanuti M, De Bardi M, Battistini L, Maccarrone M. The differential characterization of GPR55 receptor in human peripheral blood reveals a distinctive expression in monocytes and NK cells and a proinflammatory role in these innate cells. Int Immunol. 2014 doi: 10.1093/intimm/dxu097. pii: dxu097. [DOI] [PubMed] [Google Scholar]
- McHugh D, Tanner C, Mechoulam R, Pertwee RG, Ross RA. Inhibition of human neutrophil chemotaxis by endogenous cannabinoids and phytocannabinoids: evidence for a site distinct from CB1 and CB2. Mol Pharmacol. 2008;73:441–50. doi: 10.1124/mol.107.041863. [DOI] [PubMed] [Google Scholar]
- Kaufmann I, Schelling G, Eisner C, et al. Anandamide and neutrophil function in patients with fibromyalgia. Psychoneuroendocrinology. 2008;33:676–85. doi: 10.1016/j.psyneuen.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Kraft B, Wintersberger W, Kress HG. Cannabinoid receptor-independent suppression of the superoxide generation of human neutrophils (PMN) by CP55 940, but not by anandamide. Life Sci. 2004;75:969–77. doi: 10.1016/j.lfs.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Kraft B, Kress HG. Indirect CB2 receptor and mediator-dependent stimulation of human whole-blood neutrophils by exogenous and endogenous cannabinoids. J Pharmacol Exp Ther. 2005;315:641–7. doi: 10.1124/jpet.105.084269. [DOI] [PubMed] [Google Scholar]
- Montecucco F, Di Marzo V, da Silva RF, et al. The activation of the cannabinoid receptor type 2 reduces neutrophilic protease-mediated vulnerability in atherosclerotic plaques. Eur Heart J. 2012;33:846–56. doi: 10.1093/eurheartj/ehr449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard F, Lefebvre JS, Navarro P, et al. The endocannabinoid 2-arachidonoyl-glycerol activates human neutrophils: critical role of its hydrolysis and de novo leukotriene B4 biosynthesis. J Immunol. 2011;186:3188–96. doi: 10.4049/jimmunol.1002853. [DOI] [PubMed] [Google Scholar]
- Chouinard F, Turcotte C, Guan X, et al. 2-Arachidonoyl-glycerol- and arachidonic acid-stimulated neutrophils release antimicrobial effectors against E. coli S. aureus, HSV-1, and RSV. J Leukoc Biol. 2013;93:267–76. doi: 10.1189/jlb.0412200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara R, Tohyama Y, Matsusaka S, Naruse H, Kinoshita E, Tsujioka T, Katsumata Y, Yamamura H. Effects of peripheral cannabinoid receptor ligands on motility and polarization in neutrophil-like HL60 cells and human neutrophils. J Biol Chem. 2006;281:12908–18. doi: 10.1074/jbc.M510871200. [DOI] [PubMed] [Google Scholar]
- Voehringer D. Protective and pathological roles of mast cells and basophils. Nat Rev Immunol. 2013;13:362–75. doi: 10.1038/nri3427. [DOI] [PubMed] [Google Scholar]
- Jacobsen EA, Helmers RA, Lee JJ, Lee NA. The expanding role(s) of eosinophils in health and disease. Blood. 2012;120:3882–90. doi: 10.1182/blood-2012-06-330845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka S, Ikeda S, Kishimoto S, Gokoh M, Yanagimoto S, Waku K, Sugiura T. 2-Arachidonoylglycerol, an endogenous cannabinoid receptor ligand, induces the migration of EoL-1 human eosinophilic leukemia cells and human peripheral blood eosinophils. J Leukoc Biol. 2004;76:1002–9. doi: 10.1189/jlb.0404252. [DOI] [PubMed] [Google Scholar]
- Kishimoto S, Oka S, Gokoh M, Sugiura T. Chemotaxis of human peripheral blood eosinophils to 2-arachidonoylglycerol: comparison with other eosinophil chemoattractants. Int Arch Allergy Immunol. 2006;140:3–7. doi: 10.1159/000092704. [DOI] [PubMed] [Google Scholar]
- Oka S, Wakui J, Ikeda S, Yanagimoto S, Kishimoto S, Gokoh M, Nasui M, Sugiura T. Involvement of the cannabinoid CB2 receptor and its endogenous ligand 2-arachidonoylglycerol in oxazolone-induced contact dermatitis in mice. J Immunol. 2006;177:8796–805. doi: 10.4049/jimmunol.177.12.8796. [DOI] [PubMed] [Google Scholar]
- Larose MC, Turcotte C, Chouinard F, Ferland C, Martin C, Provost V, Laviolette M, Flamand N. Mechanisms of human eosinophil migration induced by the combination of IL-5 and the endocannabinoid 2-arachidonoyl-glycerol. J Allergy Clin Immunol. 2014;133:1480–1482. doi: 10.1016/j.jaci.2013.12.1081. [DOI] [PubMed] [Google Scholar]
- Facci L, Dal Toso R, Romanello S, Buriani A, Skaper SD, Leon A. Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide. Proc Natl Acad Sci U S A. 1995;92:3376–80. doi: 10.1073/pnas.92.8.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zheng J, Kulkarni A, Wang W, Garg S, Prather PL, Hauer-Jensen M. Palmitoylethanolamide regulates development of intestinal radiation injury in a mast cell-dependent manner. Dig Dis Sci. 2014;59:2693–703. doi: 10.1007/s10620-014-3212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippis D, Negro L, Vaia M, Cinelli MP, Iuvone T. New insights in mast cell modulation by palmitoylethanolamide. CNS Neurol Disord Drug Targets. 2013;12:78–83. doi: 10.2174/1871527311312010013. [DOI] [PubMed] [Google Scholar]
- Costa B, Comelli F, Bettoni I, Colleoni M, Giagnoni G. The endogenous fatty acid amide, palmitoylethanolamide, has anti-allodynic and anti-hyperalgesic effects in a murine model of neuropathic pain: involvement of CB1, TRPV1 and PPARγ receptors and neurotrophic factors. Pain. 2008;139:541–50. doi: 10.1016/j.pain.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Esposito E, Paterniti I, Mazzon E, Genovese T, Di Paola R, Galuppo M, Cuzzocrea S. Effects of palmitoylethanolamide on release of mast cell peptidases and neurotrophic factors after spinal cord injury. Brain Behav Immun. 2011;25:1099–112. doi: 10.1016/j.bbi.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Bettoni I, Comelli F, Colombo A, Bonfanti P, Costa B. Non-neuronal cell modulation relieves neuropathic pain: efficacy of the endogenous lipid palmitoylethanolamide. CNS Neurol Disord Drug Targets. 2013;12:34–44. doi: 10.2174/1871527311312010008. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Bari M, Battista N, Finazzi-Agrò A. Endocannabinoid degradation, endotoxic shock and inflammation. Curr Drug Targets Inflamm Allergy. 2002;1:53–63. doi: 10.2174/1568010023344878. [DOI] [PubMed] [Google Scholar]
- Sugawara K, Bíró T, Tsuruta D, et al. Endocannabinoids limit excessive mast cell maturation and activation in human skin. J Allergy Clin Immunol. 2012;129:726–38. doi: 10.1016/j.jaci.2011.11.009. e8. [DOI] [PubMed] [Google Scholar]
- Sugawara K, Zákány N, Hundt T, et al. Cannabinoid receptor 1 controls human mucosal-type mast cell degranulation and maturation in situ. J Allergy Clin Immunol. 2013;132:182–93. doi: 10.1016/j.jaci.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Jackson AR, Hegde VL, Nagarkatti PS, Nagarkatti M. Characterization of endocannabinoid-mediated induction of myeloid-derived suppressor cells involving mast cells and MCP-1. J Leukoc Biol. 2014;95:609–19. doi: 10.1189/jlb.0613350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluestone JA, Mackay CR, O’Shea JJ, Stockinger B. The functional plasticity of T cell subsets. Nat Rev Immunol. 2009;9:811–6. doi: 10.1038/nri2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz H, Blanco FJ, Lotz M. Anadamide, an endogenous cannabinoid receptor agonist inhibits lymphocyte proliferation and induces apoptosis. J Neuroimmunol. 1994;55:107–15. doi: 10.1016/0165-5728(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Jackson AR, Nagarkatti P, Nagarkatti M. Anandamide attenuates Th-17 cell-mediated delayed-type hypersensitivity response by triggering IL-10 production and consequent microRNA induction. PLoS ONE. 2014;9:e93954. doi: 10.1371/journal.pone.0093954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho R, Calzado MA, Di Marzo V, Appendino G, Muñoz E. Anandamide inhibits nuclear factor-κB activation through a cannabinoid receptor-independent pathway. Mol Pharmacol. 2003;63:429–38. doi: 10.1124/mol.63.2.429. [DOI] [PubMed] [Google Scholar]
- Rockwell CE, Raman P, Kaplan BL, Kaminski NE. A COX-2 metabolite of the endogenous cannabinoid, 2-arachidonyl glycerol, mediates suppression of IL-2 secretion in activated Jurkat T cells. Biochem Pharmacol. 2008;76:353–61. doi: 10.1016/j.bcp.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Joseph J, Niggemann B, Zaenker KS, Entschladen F. Anandamide is an endogenous inhibitor for the migration of tumor cells and T lymphocytes. Cancer Immunol Immunother. 2004;53:723–8. doi: 10.1007/s00262-004-0509-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfitano AM, Matarese G, Pisanti S, et al. Arvanil inhibits T lymphocyte activation and ameliorates autoimmune encephalomyelitis. J Neuroimmunol. 2006;171:110–9. doi: 10.1016/j.jneuroim.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Chiurchiù V, Catanzaro G, Borsellino G, Bernardi G, Battistini L, Maccarrone M. Anandamide suppresses proliferation and cytokine release from primary human T-lymphocytes mainly via CB2 receptors. PLoS ONE. 2010;5:e8688. doi: 10.1371/journal.pone.0008688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M, Valensise H, Bari M, Lazzarin N, Romanini C, Finazzi-Agrò A. Progesterone up-regulates anandamide hydrolase in human lymphocytes: role of cytokines and implications for fertility. J Immunol. 2001;166:7183–9. doi: 10.4049/jimmunol.166.12.7183. [DOI] [PubMed] [Google Scholar]
- Lissoni P, Tintori A, Fumagalli L, et al. The endocannabinoid anandamide neither impairs in vitro T-cell function nor induces regulatory T-cell generation. Anticancer Res. 2008;28:3743–8. [PubMed] [Google Scholar]
- Sipe JC, Arbour N, Gerber A, Beutler E. Reduced endocannabinoid immune modulation by a common cannabinoid 2 (CB2) receptor gene polymorphism: possible risk for autoimmune disorders. J Leukoc Biol. 2005;78:231–8. doi: 10.1189/jlb.0205111. [DOI] [PubMed] [Google Scholar]
- Ziring D, Wei B, Velazquez P, Schrage M, Buckley NE, Braun J. Formation of B and T cell subsets require the cannabinoid receptor CB2. Immunogenetics. 2006;58:714–25. doi: 10.1007/s00251-006-0138-x. [DOI] [PubMed] [Google Scholar]
- Rockwell CE, Snider NT, Thompson JT, Vanden Heuvel JP, Kaminski NE. Interleukin-2 suppression by 2-arachidonyl glycerol is mediated through peroxisome proliferator-activated receptor γ independently of cannabinoid receptors 1 and 2. Mol Pharmacol. 2006;70:101–11. doi: 10.1124/mol.105.019117. [DOI] [PubMed] [Google Scholar]
- Pieper K, Grimbacher B, Eibel H. B-cell biology and development. J Allergy Clin Immunol. 2013;131:959–71. doi: 10.1016/j.jaci.2013.01.046. [DOI] [PubMed] [Google Scholar]
- Sherwood TA, Nong L, Agudelo M, Newton C, Widen R, Klein TW. Identification of transcription start sites and preferential expression of select CB2 transcripts in mouse and human B lymphocytes. J Neuroimmune Pharmacol. 2009;4:476–88. doi: 10.1007/s11481-009-9169-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carayon P, Marchand J, Dussossoy D, et al. Modulation and functional involvement of CB2 peripheral cannabinoid receptors during B-cell differentiation. Blood. 1998;92:3605–15. [PubMed] [Google Scholar]
- Pereira JP, An J, Xu Y, Huang Y, Cyster JG. Cannabinoid receptor 2 mediates the retention of immature B cells in bone marrow sinusoids. Nat Immunol. 2009;10:403–11. doi: 10.1038/ni.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muppidi JR, Arnon TI, Bronevetsky Y, Veerapen N, Tanaka M, Besra GS, Cyster JG. Cannabinoid receptor 2 positions and retains marginal zone B cells within the splenic marginal zone. J Exp Med. 2011;208:1941–8. doi: 10.1084/jem.20111083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agudelo M, Newton C, Widen R, Sherwood T, Nong L, Friedman H, Klein TW. Cannabinoid receptor 2 (CB2) mediates immunoglobulin class switching from IgM to IgE in cultures of murine-purified B lymphocytes. J Neuroimmune Pharmacol. 2008;3:35–42. doi: 10.1007/s11481-007-9088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanikawa T, Kurohane K, Imai Y. Induction of preferential chemotaxis of unstimulated B-lymphocytes by 2-arachidonoylglycerol in immunized mice. Microbiol Immunol. 2007;51:1013–9. doi: 10.1111/j.1348-0421.2007.tb03985.x. [DOI] [PubMed] [Google Scholar]