Table 1. Optimization Studies for Palladium(II)-Catalyzed Oxidative Carbocyclizationsa.
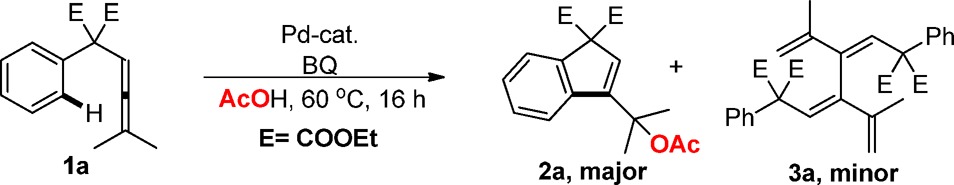
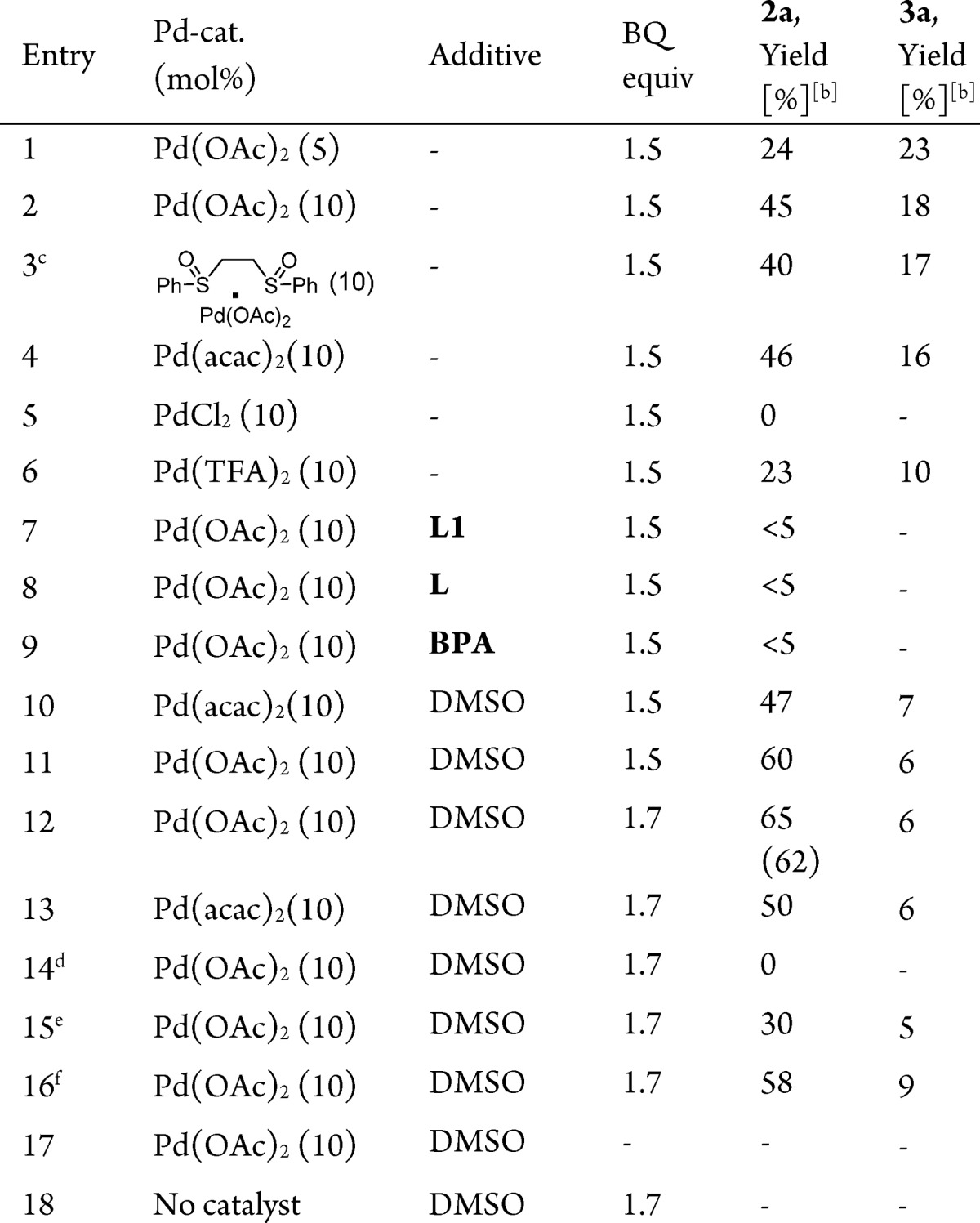
Reaction conditions: 1a (0.1 mmol), Pd-cat (5–10 mol %), additive (0.2 equiv), AcOH (1 mL), 60 °C.
Yield was determined by 1H NMR spectroscopy using mesitylene as internal standard. Parentheses represent the isolated yield. Product ratio was determined by 1H NMR spectroscopy using mesitylene as internal standard.
White catalyst.
Reaction at 25 °C.
Reaction at 40 °C.
Reaction at 80 °C. L = triphenylphosphine, L1 = acridine. BPA = BINOL−racemic phosphoric acid.
