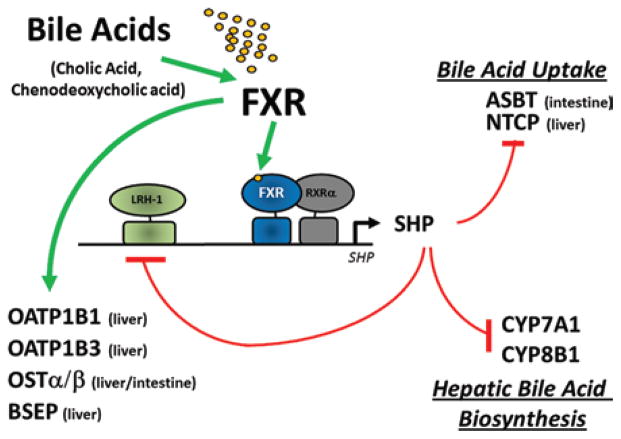
Figure 2.
Model for the FXR-mediated feed-forward and feedback regulation effects on gene expression. Feed-forward regulation by FXR in the liver and intestine is mediated by bile-acid activators of this NR superfamily member, primarily CA, and CDCA. Activated FXR, in turn, positively regulates the expression of FXR target genes that encode both drug- and bile-acid–transporter proteins OATP1B1, OATP1B3, OSTα/β, and BSEP. These transporter proteins function in the uptake and eventual elimination of drugs and bile acids from enterocytes and hepatocytes, hence the presence of excess bile acids in these tissues upregulates their own excretion and efflux. Feedback inhibition of primary bile-acid–uptake transporters ASBT and NTCP, in enterocytes and hepatocytes, respectively, is accomplished through a regulatory circuit that involves FXR-mediated induction of the expression and activity of SHP. The increased levels of the SHP nuclear receptor, which functions in a dominant-negative manner because of the lack of a functional DNA-binding domain, interacts with LRH-1 to repress the expression of genes encoding CYP7A1 and CYP8B1, enzymes centrally involved in the de novo production of additional bile acids from endogenous excess cholesterol. Increased SHP protein also represses the expression of its own gene through sequestration of LRH-1 from its own promoter. Thus, both synthesis of new bile acids from cholesterol, as well as uptake of old bile acids, is downregulated simultaneously to prevent the accumulation of excess bile acids in enterohepatic circulation.