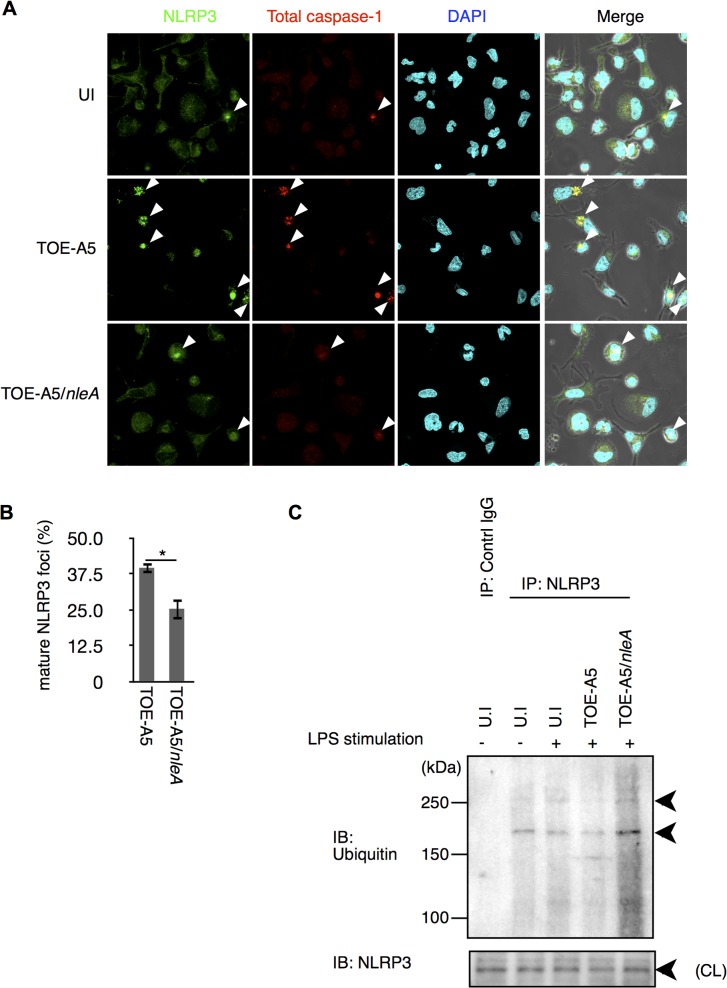
Fig 4. NleA reduces formation of mature NLRP3 inflammasomes.
(A) Mature NLRP3 foci in infected THP-1 cells. Differentiated THP-1 cells were uninfected (UI) or infected with TOE-A5 or TOE-A5/nleA. After 1 hr of infection and 3 hrs of further incubation, the cells were processed for immunostaining with an anti-NLRP3 antibody (Green), total caspase-1 (Red) and DAPI (Blue). A mature NLRP3 focus was identified as strong co-localization of the signals between NLRP3 and total caspase-1 (arrowheads). (B) Quantification of mature NLRP3 foci. Using a confocal microscope, nine random fields were investigated, and the number of mature NLRP3 foci was counted. The percentage of foci was expressed as (number of identified speck-like structures)/(number of cells in a given field of view) x100%. Student’s t-test, * p < 0.05. (C) NleA interferes with the de-ubiquitin modification of NLRP3. Differentiated THP-1 cells were primed with LPS (1 μg/ml) for 2 hrs prior to infection. Cells were then uninfected (UI) or infected with TOE-A5 or TOE-A5/nleA for 1 hr and then incubated for 3 hrs. The cell lysates were subjected to immunoprecipitation by an anti-NLRP3 antibody, and the precipitated products were analyzed by immunoblotting with an anti-ubiquitin antibody. NLPR3 in cell lysate (CL) used for immunoprecipitation was also detected.