Abstract
The propensity to attribute incentive salience to reward cues, measured by Pavlovian sign-tracking, is strongly associated with addiction-related traits including cocaine self-administration, impulsivity, novelty reactivity, and novelty preference. Despite its critical role in addiction, the genetic underpinnings of incentive salience attribution and its relationship to drug addiction are unknown. Mouse genetics can be a powerful means to discover genetic mechanisms underlying this relationship. However, feasibility of genetic dissection of sign-tracking in mice is unknown as only a single study limited to male C57BL/6J mice has rigorously examined this behavior, and limited sign-tracking was observed. Highly diverse mouse populations such as the Collaborative Cross (CC) and Diversity Outbred population (DO) possess a greater range of behavioral and genetic variation than conventional laboratory strains. In the present study, we evaluated sign-tracking and the related phenotype goal-tracking in mice of both sexes from five inbred CC and DO founder strains. Male CAST/EiJ mice exhibited robust sign-tracking; male NOD, male C57BL/6J, and female A/J mice also exhibited significant sign-tracking. Male and female mice from all strains exhibited significant goal-tracking, and significant strain and sex differences were observed. Sign-tracking in males was genetically correlated with exploration of a novel environment, and heritability of sign-tracking and goal-tracking ranged from .32 to .41. These data highlight the importance of considering genetic diversity when evaluating the occurrence of specific behavioral traits in the laboratory mouse and demonstrate that the CC and DO mouse populations can be used to discover mechanisms underlying genetic relationships among sign-tracking and addiction-related behaviors.
Keywords: Pavlovian conditioned approach, incentive salience, sign-tracking, goal-tracking, addiction, novelty, sex differences, Collaborative Cross, Diversity Outbred, mice
1. Introduction
The propensity to attribute motivational properties (i.e., incentive salience) to stimuli which predict reward is a critical component of drug addiction [1]. This phenomenon has been extensively studied in male rats using a Pavlovian conditioned approach (PCA) paradigm in which subjects learn that a conditioned stimulus, such as the extension of a lever, reliably predicts an unconditioned stimulus, such as the delivery of a food pellet [2–4]. Upon lever extension, many rats approach the pellet receptacle to await delivery of the reward, termed goal tracking (GT). Others, however, approach the lever which signals pellet-delivery, termed sign-tracking (ST). The degree to which rats attribute incentive salience to the stimulus that signals reward (i.e., manifest the ST phenotype) is strongly predictive of multiple addiction-related phenotypes [reviewed in 3] as well as phenotypes which predispose individuals to addiction such as impulsivity, novelty reactivity, and novelty preference [5–7]. Although the propensity to attribute incentive salience to reward cues is believed to be a critical component of drug addiction, the genetic underpinnings of incentive salience and its relationship to addiction are unknown. Moreover, the impact of sex on incentive salience attribution has been largely unexplored, even in the rat species [8]. As sex differences in addiction have been widely observed in humans and animal models [9], sex may be a critical variable mediating the genetic relationship between incentive salience attribution and addiction.
Mouse genetics is a powerful method for discovering the biological mechanisms driving behavioral variation, including relationships between incentive salience and addiction. Moreover, recently introduced genetically diverse experimental mouse populations such as the Collaborative Cross genetic reference panel (CC) and closely related Diversity Outbred population (DO) provide unprecedented opportunities for gene discovery [10]. However, the feasibility of using the PCA paradigm to identify the genetic underpinnings of incentive salience attribution in mice and the potential impact of sex on this relationship is unknown. This is because only a single study has rigorously examined ST and GT in the mouse species [11], and this study was limited to male mice from the C57BL/6J (B6) strain. Moreover, although Tomie and colleagues (2012) observed significant ST and GT in male B6 mice, the ST observed in that study was markedly less robust than the ST observed in many rat studies; this observation has led some to suggest that the mouse species as a whole is ill-suited for gene discovery using the PCA paradigm [12]. An alternative explanation, however, is that male mice of the B6 genotype manifest a predominantly GT phenotype, whereas other genotype-sex combinations would manifest a predominantly ST phenotype. To date, this hypothesis has not been tested.
In the present study, we assessed ST and GT using a PCA task in male (n = 48) and female (n = 48) mice from four common inbred strains (C57BL/6J, 129S1/SvImJ, AJ, NOD/ShiLtJ) and one wild-derived inbred strain (CAST/EiJ). These strains are members of the eight founder strains of the CC and DO genetic mapping populations. To enable dissociation of ST and GT from lever and pellet-dispenser approach unrelated to PCA [13], mice were tested on either a paired or unpaired version of the task. In the paired version, lever extension predicted pellet deliver. In the unpaired version, pellet-delivery was randomized. Heritability of ST and GT as well as the genetic correlation of ST and novelty reactivity were also assessed.
2. Materials and methods
2.1 Subjects
Male (n = 48) and female (n = 48) mice from four common inbred strains [C57BL/6J (B6), 129S1/SvImJ (129), A/J (AJ), NOD/ShiLtJ (NOD)] and one wild-derived inbred strain [CAST/EiJ (CAST)] were tested on the PCA task. These strains represent 5 of the 8 founder strains of the genetically diverse CC [14] and DO [15] mouse populations which we and others have used for high-resolution genetic mapping of complex behavioral traits [16–20]. All mice were acquired from The Jackson Laboratory (stock numbers 000664, 002448, 000646, 001976, and 000928, respectively) and were housed in duplex polycarbonate cages and maintained in a climate-controlled room under a standard 12:12 light-dark cycle (lights on at 0600 h). Bedding was changed weekly and mice had free access to acidified water throughout the study. Mice were provided free access to food (NIH31 5K52 chow, LabDiet/PMI Nutrition, St. Louis, MO) until behavioral testing began, at which point they were food restricted such that they weighed 85% – 90% of base weight when testing commenced each day. Mice were fed immediately following testing. A Nestlet and Shepherd Shack were provided in each cage for enrichment. Mice were housed in same sex groups of 3 – 5 prior to behavioral testing. Once testing began, mice were housed individually to facilitate food restriction. All procedures and protocols were approved by The Jackson Laboratory Animal Care and Use Committee and were conducted in compliance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
2.2 Apparatus
ST and GT data were collected using 16 Med Associates (St. Albans, Vermont) conditioning chambers (ENV-307W) enclosed in sound attenuating cubicles (ENV-022V). The floor of each chamber consisted of bars which were completely covered by a single piece of acrylic to facilitate cleaning and mouse ambulation. A retractable response lever (ENV-312-2W) was mounted to the left front wall 18 mm above the chamber floor and 28 mm away from the adjacent wall. A pellet dispenser (ENV-203-20) delivered 20 mg Bio-Serv (Flemington, NJ) Dustless Precision Pellets (F0071) into a pellet receptacle (ENV-303W). The receptacle was mounted to the right front wall 3 mm above the chamber floor, 28 mm away from the adjacent wall, and 125 mm away from the lever. A house light (ENV-315W) with bulb (CM1820; Chicago Miniature; Novi, Michigan) was mounted outside and behind the rear wall of the chamber. A monochrome micro video camera (Noldus Information Technology; Leesburg, VA) was mounted to the ceiling of the sound attenuating cubicle directly above the chamber. The camera was connected to a video capture card in a Windows PC (Noldus Information Technology) which was used to record mouse behavior during testing. Conditioning chambers were controlled by a Med Associates control unit using MED-PC IV software. The ST/GT program was written in-house in MEDState notation.
2.3 Pavlovian conditioned approach testing
Prior to testing, mice were randomly assigned to either a paired or unpaired condition (n = 9 – 10 per strain in each condition, half males and half females). Mice in the paired condition received PCA training in which pellet-delivery was paired predictably with lever extension and retraction. Specifically, pellet-delivery and lever-retraction occurred simultaneously 10 s following lever extension. Mice in the unpaired condition received identical training with the exception that pellet-delivery was randomized relative to lever extension and retraction. The house light was illuminated throughout the session. Sessions consisted of 25 trials. Each trial was followed by an inter-trial interval (ITI) of random duration ranging from 30 to 150 s. The lever was extended and retracted at the beginning and end, respectively, of each 10 s trial. For mice in the paired group, a pellet was delivered concurrently with lever-retraction. For mice in the non-paired group, a pellet was delivered at a random time between the beginning of each ITI and the end of the subsequent trial. All mice in the study received 25 pellets during each session. Mice were tested between 11 AM and 4 PM. Each chamber was cleaned using 70% ethanol between testing sessions. The number of lever presses and pellet-receptacle head entries during the trial and ITI were recorded automatically on each session by means of infrared detectors built into the levers and pellet receptacles. Because many mice showed evidence of (1) lever contact that did not result in lever depression and (2) shallow pellet-receptacle head entry that did not result in an infrared beam break, the entire session was recorded for all mice on the final testing day (session 15), and video data were subsequently scored to quantify approach to the lever and pellet receptacle during the trial and ITI on this session.
2.4 Exploration of a novel open field
Open field testing was performed in a separate cohort of mice (N = 80, 8 males and 8 females per strain) and was collected as part of a larger QTL mapping study of novelty-, anxiety-, depression-, and impulsivity-related traits. These data have been published separately [19] and are publicly available from the Mouse Phenome Database at The Jackson Laboratory (http://phenome.jax.org/; accession ID: Chesler4). The open-field arena (39 × 39 × 39 cm) was composed of white opaque acrylic walls and a dark gray acrylic floor, was located in a 10 × 15 foot room, and was illuminated at 43 ± 4 lux. Mice were individually placed into the center of the arena and allowed to explore for 20 min. Total distance traveled over the 20 min testing session was recorded.
2.5 Dependent variables
Using infrared beam break data, ST rate and GT rate were calculated as number of lever presses and pellet-receptacle head-entries, respectively, per second for each trial on all 15 sessions. GT rate was calculated for both the trial and ITI, but ST could only be calculated for trials because the lever was retracted during the ITI. On the final session, ST and GT were scored by video analysis and were operationally defined as the duration (s) of lever contact and the duration of head entry into the pellet receptacle, respectively. Duration of ST and GT were scored for each of the trials and ITIs on the final testing session using the Observer XT software package (Noldus Information Technology). During a trial, ST was scored whenever contact between the lever and nose or lever and paw was observed. During the ITI, ST was scored when the mouse made nose- or paw-contact with the lever housing directly in front of the retracted lever. ST was not scored when incidental contact with the lever occurred (e.g., backing into the lever). GT was scored when any part of the nose was inside the food receptacle. An ST:GT ratio was calculated on the final session for each mouse by dividing the total duration of ST during all trials by the total duration of GT during all trials. For open field testing, total distance traveled during the 20 min testing session was considered an index of novel environment exploration.
2.6 Statistical analysis
Normality and linearity of dependent measures were assessed by inspection of normal probability plots and scatterplots. Z-scores and Mahalanobis distance scores were examined to identify univariate and multivariate outliers, respectively. Z-scores in excess of the absolute value of 3.29 (outlier at p < .001, two-tailed test) and extreme Mahalanobis distance scores (outlier at p < .001, χ2 test) were considered significant outliers. Data which violated statistical assumptions were square root or log base 10 transformed using methods recommended by Tabachnick and Fidell [21]. Repeated measures analysis of variance (ANOVA) or covariance (ANCOVA) was used to assess group differences on ST and GT measures. The assumption of homogeneity of variance across groups was assessed using Mauchly’s test of sphericity. When this assumption was violated, the Huynh–Feldt correction was used. All data will be available in the Mouse Phenome Database (http://phenome.jax.org).
2.7 Attrition
During the course of testing one male NOD mouse developed symptoms of insulin-dependent diabetes mellitus, common in this strain, and did not complete the study. Data for this mouse was excluded from statistical analysis. All other mice were healthy throughout the study.
3. Results
3.1 Acquisition of sign- and goal-tracking across 15 testing sessions
3.1.1 Acquisition of goal-tracking
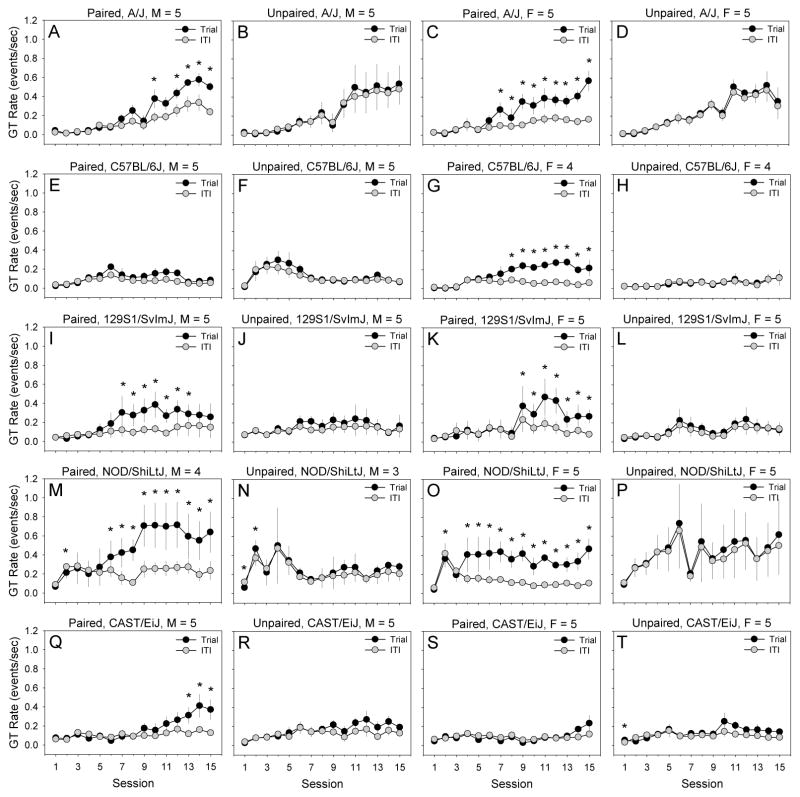
We performed a 2 × 2 × 2 × 5 × 15 mixed-model ANOVA using rate of head entries into the food-receptacle (quantified using IR beam breaks) as an index of GT. Between-subjects factors were pairing of lever-extension and pellet-delivery (paired, unpaired), lever-extension (extended, retracted), sex, and strain (AJ, B6, 129, NOD, CAST). Session (1 – 15) was a within-subjects factor. The between-subjects factor “lever extension” allowed for the dissociation of behavior which occurred during trials (i.e., lever was extended) and ITIs (i.e., lever was retracted). A statistically significant 5-way interaction of strain, sex, lever-extension, lever-pellet pairing, and session was detected [F (56, 243) = 1.52, p < .05]. To determine the nature of this interaction, we examined performance of male and female mice of each strain in the paired and unpaired conditions across the 15 testing sessions during the trial and ITI (Fig. 1). With the exception of male B6 mice and female CAST mice, male and female mice from all strains in the paired group exhibited acquisition of the GT response as indicated by a significantly greater GT rate during the trial relative to the ITI (Fig. 1A, I, M, Q, C, G, K, O). By contrast, GT rate during the trial and ITI did not differ in the unpaired group in male (Fig 1B, F, J, N, R) or female (Fig 1D, H, L, P, T) mice of any strain.
Figure 1. Development of goal-tracking across sessions in male (M) and female (F) mice from the A/J, C57BL/6J, 129S1/SvImJ, NOD/ShiLtJ, and CAST/EiJ inbred strains.
With the exception of male C57BL/6J (E) and female CAST/EiJ mice (S), male (A, I, M, Q) and female mice (C, G, K, O) from all strains in the paired group developed the goal-tracking response as indicated by a significantly greater goal-tracking rate during trials relative to inter-trial intervals. In contrast, GT rate during trials and inter-trial intervals did not differ in the unpaired group in male (B, F, J, N, R) or female (D, H, L, P, T) mice of any strain. Goal-tracking rate was quantified using infrared beam breaks.
* p < .05
3.1.2 Acquisition of sign-tracking
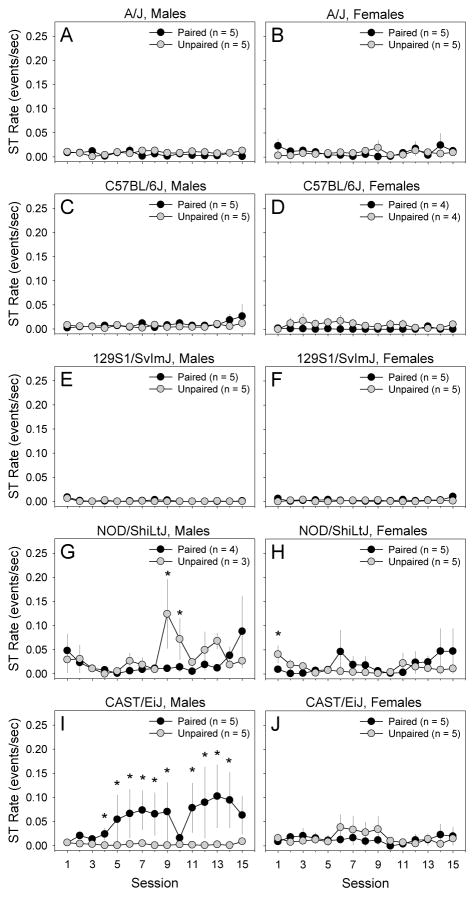
We performed a 2 × 2 × 5 × 15 mixed-model ANOVA using lever pressing rate (quantified using IR beam breaks) as an index of ST. Between-subjects factors were pairing of lever-extension and pellet-delivery, sex, and strain. Session was a within-subjects factor. We could not dissociate between lever pressing during the trial and ITI because the lever was retracted during the ITI. A statistically significant 4-way interaction of strain, sex, lever-pellet pairing, and session was detected [F (56, 243) = 1.47, p < .05]. To determine the nature of this interaction, we examined performance of male and female mice of each strain in the paired and unpaired conditions across the 15 testing sessions (Fig. 2). Male CAST mice in the paired conditioned exhibited a significantly greater ST rate relative to mice in the unpaired condition (Fig. 2I) indicating acquisition of the ST response. Male and female mice in other strains did not exhibit statistically significant ST behavior when lever pressing rate was used as the index of ST.
Figure 2. Development of sign-tracking across sessions in male (M) and female (F) mice from the A/J, C57BL/6J, 129S1/SvImJ, NOD/ShiLtJ, and CAST/EiJ inbred strains.
Male CAST/EiJ mice in the paired condition developed the sign-tracking response as indicated by a significantly greater sign-tracking rate relative to control mice in the unpaired condition (I). Sign-tracking rate of male and female mice in other strains did not differ significantly from that of unpaired controls (A, B, C, D, E, F, G, H, J). Sign-tracking rate was quantified using infrared beam breaks.
* p < .05
3.2 Sign- and goal-tracking duration on the final testing session assessed by video analysis
3.2.1 Goal-tracking duration
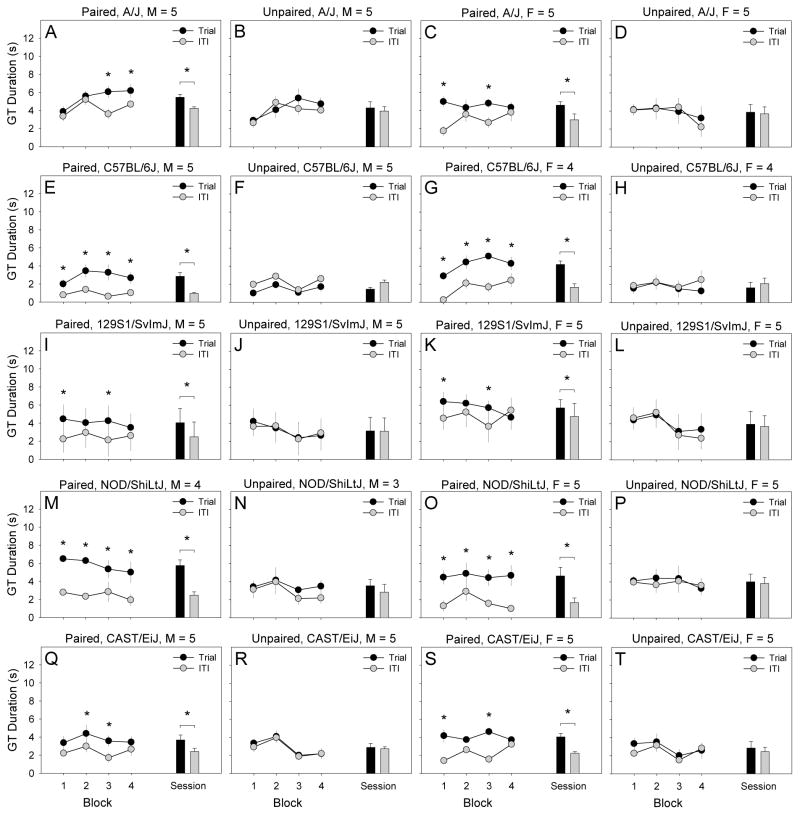
We performed a 2 × 2 × 2 × 5 × 25 mixed-model ANCOVA using GT duration (quantified using video analysis) as the dependent measure. Between-subjects factors were pairing of lever-extension and pellet-delivery, lever-extension, sex, and strain. Trial (1 – 25) was a within-subjects factor, and chamber was a covariate. A statistically significant 4-way interaction of strain, lever-extension, lever-pellet pairing, and trial was detected [F (96, 1728) = 1.35, p < .05], and the 5-way interaction of strain, sex, lever-extension, lever-pellet pairing, and trial approached significance [F (96, 1728) = 1.25, p = .05]. To determine the nature of these interactions, we examined GT duration of male and female mice from each strain in the paired and unpaired conditions separately during the final testing session (Fig. 3). Male (Fig. 3A, E, I, M, Q) and female (Fig. 3C, G, K, O, S) mice of all strains in the paired group exhibited statistically significant GT as indicated by a significantly greater GT duration during trials relative to ITIs. By contrast, GT duration during trials and ITIs did not differ in male (Fig 3B, F, J, N, R) or female (Fig 3D, H, L, P, T) mice of any strain in the unpaired group.
Figure 3. Goal-tracking duration on the final testing session in male (M) and female (F) mice from the A/J, C57BL/6J, 129S1/SvImJ, NOD/ShiLtJ, and CAST/EiJ inbred strains.
Male (A, E, I, M, Q) and female (C, G, K, O, S) mice of all strains in the paired group exhibited goal-tracking as indicated by a significantly greater goal-tracking duration during trials relative to inter-trial intervals. By contrast, goal-tracking duration during trials and inter-trial intervals did not differ in male (B, F, J, N, R) or female (D, H, L, P, T) mice of any strain in the unpaired condition. Goal-tracking duration on the final session was quantified by video analysis.
* p < .05
3.2.2 Sign-tracking duration
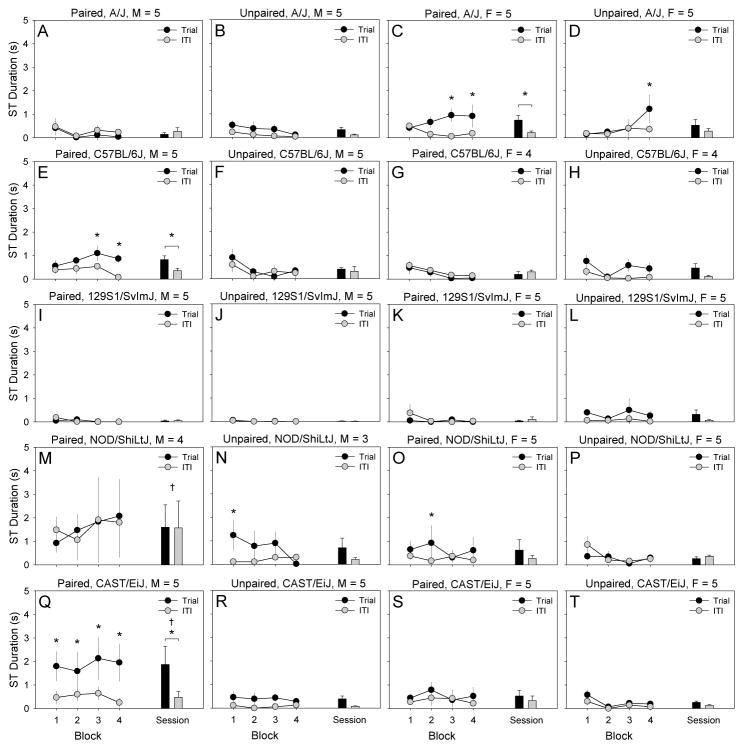
We performed a 2 × 2 × 2 × 5 × 25 mixed-model ANCOVA using ST duration (quantified using video analysis) as an index of ST. Between-subjects factors were pairing of lever-extension and pellet-delivery, lever-extension, sex, and strain. Trial was a within-subjects factor, and chamber was a covariate. Statistically significant 4-way interactions of strain, sex, lever-extension, and lever-pellet pairing [F (4, 72) = 2.71, p < .05] as well as sex, lever-extension, lever-pellet pairing, and trial [F (24, 1728) = 1.57, p < .05] were detected. To determine the nature of these interactions, we examined ST duration of male and female mice from each strain in the paired and unpaired conditions separately (Fig. 4).
Figure 4. Sign-tracking duration on the final testing session in male (M) and female (F) mice from the A/J, C57BL/6J, 129S1/SvImJ, NOD/ShiLtJ, and CAST/EiJ inbred strains.
Male CAST/EiJ mice in the paired condition (Q) exhibited robust sign-tracking as indicated by a significantly greater sign-tracking duration relative to unpaired controls (R) and during trials relative to inter-trial intervals. Male NOD/ShiLtJ mice in the paired condition (M) exhibited robust sign-tracking as indicated by a significantly greater sign-tracking duration relative to unpaired controls (N). In contrast to male CAST/EiJ mice, male NOD/ShiLtJ mice in the paired condition approached and attempted to reach the retracted lever during inter-trial intervals as indicated by a significantly greater sign-tracking duration during inter-trial intervals relative to that of unpaired controls (N). Male C57BL/6J (E) and female A/J (C) mice in the paired condition exhibited a significantly greater sign-tracking duration during trials relative to inter-trial intervals. This indicates significant but less robust sign-tracking in male C57BL/6J and female A/J mice relative to male CAST/EiJ and NOD/ShiLtJ mice. Mice from other strain-sex subgroups in the paired condition and mice in the unpaired condition did not exhibit significant sign-tracking. Sign-tracking duration on the final session was quantified by video analysis.
* p < .05, trial vs ITI
† p < .05, paired vs unpaired
ST duration of male CAST mice in the paired condition was significantly greater (p < .05) during trials than ITIs (Fig. 4Q), and ST duration during trials was significantly greater (p < .05) in male CAST mice in the paired condition relative to male CAST mice in the unpaired condition (Fig. 4R). Collectively, these data indicate robust ST in male CAST mice. ST duration of male NOD mice in the paired group was significantly greater (p < .05) than ST duration of male NOD mice in the unpaired group (Fig 4M, N). However, ST duration of male NOD mice in the paired group was elevated during both the trial and ITI (Fig 4M). Collectively, these data indicate that male NOD mice exhibited statistically significant ST and continued to approach the food-paired lever even after it was retracted. ST duration of male B6 mice (Fig. 4E) and female AJ mice (Fig. 4C) in the paired group was significantly greater (p < .05) during trials than ITIs, suggesting some degree of ST in these mice. ST duration during trials and ITIs did not differ in male (Fig 4B, F, J, N, R) or female (Fig 4D, H, L, P, T) mice of any strain in the unpaired group.
3.2.3 Strain and sex differences in sign- and goal-tracking duration
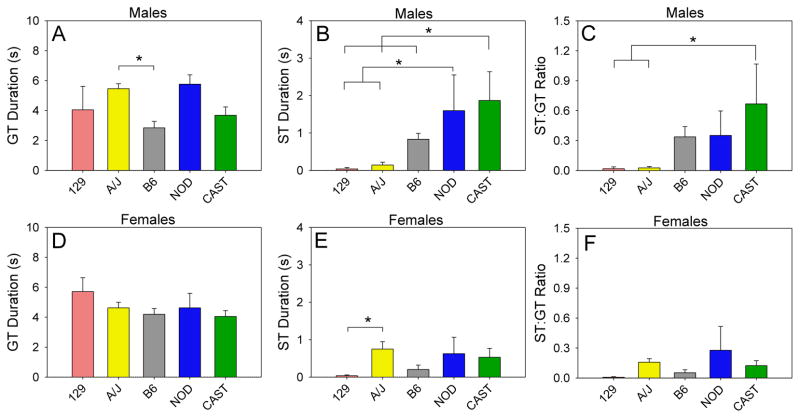
The range of GT duration was greater in males (Fig 5A) than females (Fig 5D). Only male AJ and male B6 strains differed significantly (p < .05). The range of ST duration was also greater in males (Fig 5B) than females (Fig 5E). ST duration of male CAST mice was significantly greater (p < .05) than that of male 129, AJ, and B6 mice (Fig 5B), and ST duration of male NOD mice was significantly greater (p < .05) than those of male 129 and AJ mice (Fig 5B). ST duration of female AJ mice was significantly greater (p < .05) than that of female 129 mice (Fig 5E). The range of the ST:GT duration ratio was greater in males (Fig 5C) than females (Fig 5F). The ST:GT duration ratio of male CAST mice was significantly greater (p < .05) than those of male 129 and AJ mice (Fig 5C). ST:GT duration ratios of female mice did not differ significantly (Fig 5F).
Figure 5. Strain and sex differences in sign- and goal-tracking.
The range of goal-tracking duration across strains was greater in males (A) than females (D), and only male A/J and male C57BL/6J strains differed significantly. The range of sign-tracking duration across strains was also greater in males (B) than females (E). In males, CAST/EiJ mice exhibited the greatest degree of sign-tracking, and sign-tracking in these mice was significantly greater than that of 129S1/SvImJ, A/J, and C57BL/6J mice. In females, A/J mice exhibited the greatest degree of sign-tracking, and sign-tracking in these mice was significantly greater than that of 129S1/SvImJ mice. Male CAST/EiJ mice exhibited the greatest ST:GT duration ratio, and this ratio differed significantly from those of male 129S1/SvImJ and A/J mice (C). ST:GT duration ratios of female mice did not differ significantly (F).
* p < .05
3.3 Genetic correlation of sign-tracking and exploratory behavior
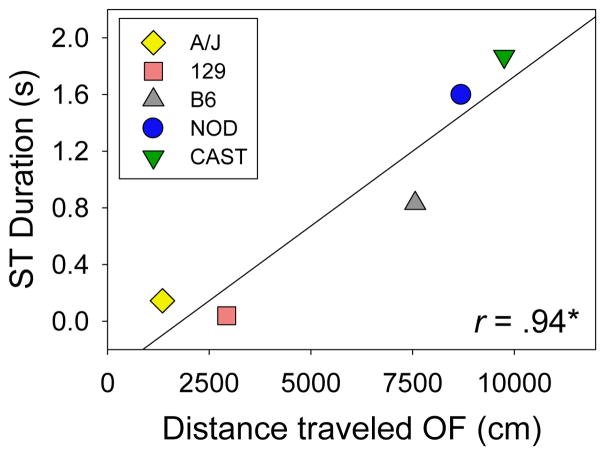
We assessed the genetic correlation between sign-tracking and exploration of a novel open field independently in male and female mice. A strong and statistically significant genetic correlation (r = .94, p < .05) between sign-tracking and distance traveled (cm) during a single 20 min session in a novel open field was observed in male mice (Fig 6). To exclude the possibility that this relationship was an artifact of strain-dependent differences in distance traveled in the conditioning chamber, we examined the genetic correlation between distance traveled in the open field and the difference between ST in the paired group and ST in the unpaired group: this genetic relationship was also strong and significant (r = .93, p < .05). We did not observe a genetic correlation between ST and distance traveled in a novel open field in females, nor was a genetic correlation observed between GT and distance traveled in a novel open field in males or females (Fig S1).
Figure 6. Genetic correlation of sign-tracking and distance traveled in a novel open field in male A/J, C57BL/6J, 129S1/SvImJ, NOD/ShiLtJ, and CAST/EiJ inbred mouse strains.
A strong genetic correlation (r = .94, p < .05) between sign-tracking and distance traveled (cm) during a 20 min session in a novel open field was observed in male, but not female mice. Open field data (8 males per strain) were collected from a separate cohort of mice. A genetic correlation between goal-tracking and distance traveled in the open field was not observed in male or female mice.
3.4 Heritability of sign-tracking and goal-tracking
Heritability estimates of ST and GT ranged from .32 to .41 (Table 1) and were calculated using mice from the paired group. Heritability was defined as the ratio of between-strain variance to the total within- plus between-strain variance, excluding variance accounted for by cohort and chamber.
Table 1.
Heritability estimates of sign-tracking and goal-tracking duration
| Trait | Heritability
|
|
|---|---|---|
| Males | Females | |
| ST duration (s/trial) | 0.35 | 0.32 |
| GT duration (s/trial) | 0.34 | 0.41 |
4. Discussion
In the present study, we have demonstrated for the first time robust strain and sex differences in Pavlovian ST in the mouse species. Male mice from the CAST (Fig 4Q), NOD (Fig 4M), and B6 (Fig 4E) inbred strains and female mice from the AJ inbred strain (Fig 4C) exhibited statistically significant ST on the final testing session. Across strains, males relative to females exhibited the greatest ST range (Fig 5B vs Fig 5E), and male CAST mice exhibited the most robust ST of all sex-strain groups (Fig 4Q). In contrast to the robust ST observed in male CAST mice, female CAST mice failed to exhibit statistically significant ST (Fig 4S). Males and females from all strains exhibited statistically significant GT on the final testing session (Fig 3), and GT was evident in males and females from most strains during acquisition sessions (Fig 1). Finally, a strong positive genetic correlation between ST and novelty reactivity was observed in male, but not female mice (Fig 6).
4.1 Sign-tracking and goal-tracking: strain and sex differences in five inbred mouse strains
Although past studies have examined Pavlovian conditioning in mice [22–24], only one has rigorously examined ST and GT in mice using both a paired condition and an unpaired control condition [11]. This design is critical for dissociating PCA from lever and pellet-dispenser approach due to other factors, such as the propensity to approach or avoid salient stimuli, or differences in general activity [13]. Using this strategy, Tomie and colleagues (2012) observed significant ST and GT in male mice from the B6 inbred background. Findings from the present study confirm the presence of ST in B6 males (Fig. 4E) and, considering the absence of ST in B6 females (Fig. 4G), provide novel evidence that ST propensity in the B6 strain is sex dependent. In contrast to ST propensity, both male and female mice from the B6 strain exhibited statistically significant GT (Fig. 3E, G), and the degree of GT propensity was equivalent in males and females. This observation excludes the possibility of an impairment in PCA learning and suggests instead that the observed sex difference in ST propensity in B6 mice reflects a true difference in the propensity to attribute incentive salience to reward paired stimuli.
In addition to confirming the existence of ST in male B6 mice and revealing sex dependence of the ST trait in this strain, we provide novel evidence of significant strain differences in ST and GT propensity across five of the inbred founder strains of the DO and CC genetic mapping populations. Moreover, as in the B6 strain, we show clear sex dependence of ST propensity in CAST, NOD, and AJ strains. CAST (Fig 4Q) and NOD (Fig 4M) males exhibited strong ST propensity, but females from these two strains did not exhibit significant ST (Fig 4S, O). Conversely, females from the AJ strain exhibited significant ST (Fig 4C), but male AJ mice did not (Fig 4A). Neither male nor female mice from the 129 strain exhibited significant ST (Fig 4I, K). Importantly, as in the B6 strain, both males and females from the CAST, NOD, AJ, and 129 strains exhibited significant GT behavior relative to unpaired controls (Fig 3).
Manifestation of ST in NOD males was unique in that these mice approached the retracted lever during ITIs as well as during trials (Fig 4M). NOD males appeared highly motivated to make contact with the lever during ITIs and would persistently and vigorously reach into the lever housing and touch the retracted lever. In contrast, mice from other strains largely restricted lever approach to trials (i.e., when the lever was extended).
In the present study, mice were food restricted and individually housed at testing outset. Mice were group housed prior to the study and were provided access to enrichment devices (i.e., Nestlet and Shepherd Shack) throughout life. In this regard, environmental factors have been shown to influence ST propensity in rats [25–28], and specific genotypes may confer an elevated risk to develop ST behavior following certain environmental events. Consequently, environmental factors should be considered when interpreting findings from the present study.
Collectively, these data reveal a spectrum of sex-dependent ST propensity across the CC and DO founder strains assessed in the present study (Fig 5), with CAST males exhibiting the greatest propensity to exhibit the ST phenotype, and male and female 129 the least. It remains possible that mice from the sex-strain subgroups which failed to exhibit significant ST possess the capacity, albeit a relatively lower propensity, to manifest ST behavior, and that this could be quantified with a larger sample size. Manipulation of task parameters such as introducing reward uncertainty [29] or allowing more sessions for mice to develop the ST phenotype may also enhance the ability to detect ST without increasing sample size. Indexing Pavlovian to instrumental transfer may also facilitate detection of incentive salience attribution in mice. Some studies using large numbers of outbred rats (i.e., a large number of genotypes) have observed an even broader spectrum of the ST and GT traits [30]. Specifically, at the extreme ranges of the STGT spectrum some animals spend the entire duration of the trial sign-tracking whereas others spend the entire duration of the trial goal-tracking. Importantly, the extreme phenotypes observed in these studies are a consequence of the vast number of unique allelic combinations which can occur in outbred animals; we observe this same phenomenon, referred to as “transgressive segregation”, in DO mice relative to their inbred founders [19, 20]. Thus, it is likely that the full spectrum of ST and GT would be observed in a larger study of DO mice and that the genes and mechanisms driving these behaviors could be identified.
4.2 Genotype-dependent idiosyncrasies in the manifestation of sign-tracking and goal-tracking
Although attribution of incentive salience to a reward paired stimulus is generally regarded as increasing the propensity to approach that stimulus [3], the majority of PCA studies in rats have quantified ST not as approach or contact with a reward paired lever, but rather as depression of the lever. While convenient, this strategy may result in a somewhat imprecise measurement of incentive salience attribution as there is no a priori reason to suggest that an animal which is driven to approach a reward paired lever will also be driven to press that lever. In this regard, Tomie and colleagues observed that male B6 mice would often approach but not depress the reward paired lever [11], and the existence of ST behaviors directed towards the lever which are not captured by lever pressing has been documented in rat studies [29, 31]
In the present study, only CAST males exhibited unambiguous acquisition of the ST response when lever pressing rate was used as the ST index (Fig 2I). Using these data alone, it could be argued that only CAST males exhibited the ST response. However, it seems more likely that NOD males, B6 males, and AJ females did acquire the ST response, but that this response was best captured on the final session (Fig 4M, E, C) when ST was operationalized as duration of lever contact (quantified by video analysis), not during earlier sessions (Fig 2G, C, B) when ST was operationalized as rate of lever depression (quantified automatically by infrared beam breaks). With respect to this, video analysis indicated that the ST response in mice was manifested almost exclusively by approaching and exploring the lever with the nose, not pressing the lever as would be observed in an operant conditioning paradigm. Interestingly, in the present study we observed that this pattern of lever exploration was very strain specific, and subtle differences in lever exploration patterns determined whether lever depression occurred secondary to lever exploration. For example, male NOD mice would vigorously explore all sides of the lever with their nose and would bite the lever while doing so. During ITIs when the lever was retracted, NOD males would attempt to reach into the housing and access the lever. Although relatively aggressive and frenzied, the lever exploration which was characteristic of male NOD mice rarely resulted in lever depression and, consequently, was not detected through automated means. Conversely, although male CAST mice were relatively less frenzied in their lever exploration (e.g., they did not bite the lever and did not attempt to access the lever through the lever housing during ITIs), the lever exploration which was characteristic of CAST mice often did result in lever depression due to subtle differences in pattern and direction of head movements. We observed similar strain-specific ST patterns in male B6 and female AJ mice. Although less common, we also observed a similar phenomenon affecting the automated quantification of GT in which mice would approach the pellet receptacle and look for a pellet without entering far enough to break the infrared beam. Collectively, these observations suggest that due to genotype-dependent idiosyncrasies in the manifestation of ST and GT, special care must be taken when using these behaviors to index incentive salience attribution.
4.3 Genetic correlation of sign-tracking and exploration of a novel environment in male mice
We observed in male but not female mice a strong positive genetic correlation between ST and distance traveled in a novel open field (i.e., novelty reactivity) (Fig 6). The relationship between ST and novelty reactivity was observed when using ST scores of the paired group and when using the difference between ST scores of the paired and unpaired groups; this observation indicates that the association between ST and novelty-reactivity was not simply an artifact of increased activity in the conditioning chamber in the high-ST strains.
The genetic relationship between ST and novelty reactivity has been consistently observed in rat lines selectively bred for high novelty reactivity [2, 5]. Interestingly, these lines exhibit other addiction-related behaviors including rapid acquisition of intravenous cocaine self-administration (IVSA) and elevated levels of impulsivity [reviewed in 32], suggesting shared genetic mechanisms among these traits. Beckmann and colleagues [6] recently examined the phenotypic relationship between ST and novelty-related traits in outbred rats and detected a positive relationship between ST and novelty preference. Novelty preference, which is phenotypically [33, 34] and genetically [35] dissociable from novelty reactivity in rodents, has also been shown to covary positively with ST and cocaine IVSA [6], and we have previously observed a positive association between multiple novelty-related traits and cocaine IVSA in male and female DO mice [36]. Moreover, the strain distribution of ST propensity observed in the present study is similar to that of holeboard exploration (an index of novelty seeking) [35]. Collectively, these findings suggest overlapping neurogenetic mechanisms driving ST and other addiction-related traits.
Interestingly, although Beckmann and colleagues [6] detected a relationship between novelty and sign-tracking in the form of novelty preference, they did not detect a relationship between ST and novelty reactivity. One explanation for this is that the outbred rats used in the Beckmann study (Sprague-Dawley) lacked the allelic diversity to manifest a relationship between ST and novelty reactivity. In this regard, significant variation in ST and GT has been observed in Sprague-Dawley rats originating from different vendors as well as from individual colonies operated by the same vendor, and these phenotypic differences are likely due to limited genetic diversity stemming from population bottlenecks within these colonies [37]. Thus, it is likely that some outbred rat stocks do not possess the allelic diversity necessary to exhibit robust relationships among all addiction related traits that would be observed in a genetically diverse population such as the DO or CC.
4.4 The Collaborative Cross and Diversity Outbred population as resources for the discovery of genes underlying the relationship between sign-tracking and addiction-related traits
The Collaborative Cross (CC) is a multiparental genetic reference population developed to overcome the limitations of existing genetic reference populations including limited genetic diversity, inconsistent genetic architecture, and high linkage disequilibrium [14, 38]. The CC was founded from eight inbred mouse strains consisting of five classical strains (AJ, C57BL/6J, 129S1/SvImJ, NOD/LtJ, NZO/HILtJ) and three wild-derived strains (CAST/EiJ, PWK/PhJ, WSB/EiJ). The closely related Diversity Outbred population (DO) was founded from incipient CC strains and is maintained using a randomized outbreeding strategy [15, 20]. The DO segregates the same allelic variants as the CC but in a different genetic architecture. Specifically, because DO mice are outbred, each mouse is a unique individual containing one of a vast combination of alleles from the eight founder strains. Importantly, the eight CC founders represent 89% of the genetic diversity in the mouse genome [39]. Consequently, the CC and DO contain substantially more genetic variation than all other experimental mouse populations. Collectively, the CC and DO offer several advantages over conventional genetic mapping populations including greater genetic diversity, higher-precision quantitative trait locus (QTL) mapping, reduced linkage disequilibrium, and a restoration of the broad and continuous range of behavioral phenotypes which have been constrained in classical inbred strains and conventional genetic mapping populations [19].
Mice tested in the present study represent five of the eight CC and DO founder strains, and data presented here reveal a spectrum of ST and GT propensity across these strains. Heritability estimates of .32 – .41 indicate the feasibility of mapping the ST and GT traits in the CC and DO. In this regard, we have recently used the DO to map high-precision QTL for several novelty- and anxiety-related traits, many of them with heritability estimates below those we observed for ST and GT in the present study [19]. Notably, these QTL had narrow support intervals ranging from 1 to 3 Mb in size containing relatively few genes (half had ≤21 candidates). Half of these QTLs were associated with wild-derived alleles, demonstrating the value of added genetic diversity in the DO and CC.
The strong genetic correlation between novelty reactivity in the open field and ST in male CC and DO founders (Fig. 6) suggests that the genetic mechanisms underlying this relationship can be mapped in the CC and DO. The association of novelty-related traits and ST may be particularly useful in the search for genes and mechanisms underlying drug addiction because prior studies in rats have identified strong interrelationships between ST, cocaine IVSA, and novelty-related traits [6, 32]. Independent studies examining the association of novelty- and addiction-related traits using cocaine IVSA assays with high relevance to the addiction construct confirm the relationship between these phenotypes [reviewed in 33]. Importantly, we have recently identified a strong positive relationship between multiple novelty-related traits and cocaine IVSA in a large sample of DO mice [36]. Combined with results from the present study, this suggests that the relationships between ST, cocaine IVSA, and novelty-related traits can be mapped in the DO and CC populations. This approach enables identification of unknown and perhaps unexpected genes and mechanisms responsible for the loss of control over drug taking and drug seeking which, ultimately, drive drug addiction.
5. Conclusion
The propensity to attribute incentive salience to reward cues plays a critical role in drug addiction, and discovery of the genetic mechanisms driving this relationship could lead to a greater understanding of addiction liability. Mouse genetics provides powerful resources to study this phenomenon; however, there has been limited evidence that mice exhibit sufficiently robust sign-tracking for genetic analysis. In the present study, we have shown heritable variation in the propensity to attribute incentive salience to reward cues (i.e., sign-tracking) in founder strains of the Collaborative Cross and Diversity Outbred population. This effect was sex dependent. In males, sign-tracking was strongly genetically correlated with novelty reactivity, a trait which has been associated with an addiction-prone phenotype. These data highlight the importance of considering genetic diversity when evaluating the occurrence of specific behavioral traits in the laboratory mouse. Collectively, these findings are consistent with previous studies indicating the utility of the Collaborative Cross and Diversity Outbred population for uncovering genes and mechanisms underlying complex relationships between addiction and addiction-related traits.
Supplementary Material
Genetic association of distance traveled in a novel open field and sign- and goal-tracking in male and female A/J, C57BL/6J, 129S1/SvImJ, NOD/ShiLtJ, and CAST/EiJ inbred mouse strains.
We detected Pavlovian conditioned approach in Collaborative Cross and DO founders
Significant strain differences in sign-tracking and goal-tracking were observed
Strain differences in sign-tracking and goal-tracking interacted with sex
Sign-tracking and novelty reactivity were genetically correlated in males
Sign-tracking was robust in some mouse strains
Acknowledgments
We would like to thank Dr. Shelly Flagel for helpful advice during the early stages of this project. We would also like to thank Katie Toal and Andrew Gallup for assistance with data collection and analysis. The authors gratefully acknowledge support from The Jackson Laboratory, NIDA DA37927, and NIGMS GM76468. KAM was supported by the Research Experience for Undergraduates Site at the Jackson Laboratory (DBI-1262049).
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2008;363:3137–46. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, et al. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–7. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson TE, Yager LM, Cogan ES, Saunders BT. On the motivational properties of reward cues: Individual differences. Neuropharmacology. 2014;76(Pt B):450–9. doi: 10.1016/j.neuropharm.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomie A, Grimes KL, Pohorecky LA. Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain research reviews. 2008;58:121–35. doi: 10.1016/j.brainresrev.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, et al. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beckmann JS, Marusich JA, Gipson CD, Bardo MT. Novelty seeking, incentive salience and acquisition of cocaine self-administration in the rat. Behavioural brain research. 2011;216:159–65. doi: 10.1016/j.bbr.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lovic V, Saunders BT, Yager LM, Robinson TE. Rats prone to attribute incentive salience to reward cues are also prone to impulsive action. Behavioural brain research. 2011;223:255–61. doi: 10.1016/j.bbr.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pitchers KK, Flagel SB, O’Donnell EG, Solberg Woods LC, Sarter M, Robinson TE. Individual variation in the propensity to attribute incentive salience to a food cue: Influence of sex. Behavioural brain research. 2014;278C:462–9. doi: 10.1016/j.bbr.2014.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becker JB, Hu M. Sex differences in drug abuse. Frontiers in neuroendocrinology. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chesler EJ. Out of the bottleneck: the Diversity Outcross and Collaborative Cross mouse populations in behavioral genetics research. Mammalian genome : official journal of the International Mammalian Genome Society. 2014;25:3–11. doi: 10.1007/s00335-013-9492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomie A, Lincks M, Nadarajah SD, Pohorecky LA, Yu L. Pairings of lever and food induce Pavlovian conditioned approach of sign-tracking and goal-tracking in C57BL/6 mice. Behavioural brain research. 2012;226:571–8. doi: 10.1016/j.bbr.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parker CC, Chen H, Flagel SB, Geurts AM, Richards JB, Robinson TE, et al. Rats are the smart choice: Rationale for a renewed focus on rats in behavioral genetics. Neuropharmacology. 2014;76(Pt B):250–8. doi: 10.1016/j.neuropharm.2013.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rescorla RA. Pavlovian conditioning and its proper control procedures. Psychological review. 1967;74:71–80. doi: 10.1037/h0024109. [DOI] [PubMed] [Google Scholar]
- 14.Churchill GA, Airey DC, Allayee H, Angel JM, Attie AD, Beatty J, et al. The Collaborative Cross, a community resource for the genetic analysis of complex traits. Nature genetics. 2004;36:1133–7. doi: 10.1038/ng1104-1133. [DOI] [PubMed] [Google Scholar]
- 15.Churchill GA, Gatti DM, Munger SC, Svenson KL. The Diversity Outbred mouse population. Mammalian genome : official journal of the International Mammalian Genome Society. 2012;23:713–8. doi: 10.1007/s00335-012-9414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Philip VM, Sokoloff G, Ackert-Bicknell CL, Striz M, Branstetter L, Beckmann MA, et al. Genetic analysis in the Collaborative Cross breeding population. Genome Res. 2011;21:1223–38. doi: 10.1101/gr.113886.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aylor DL, Valdar W, Foulds-Mathes W, Buus RJ, Verdugo RA, Baric RS, et al. Genetic analysis of complex traits in the emerging Collaborative Cross. Genome research. 2011;21:1213–22. doi: 10.1101/gr.111310.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Recla JM, Robledo RF, Gatti DM, Bult CJ, Churchill GA, Chesler EJ. Precise genetic mapping and integrative bioinformatics in Diversity Outbred mice reveals Hydin as a novel pain gene. Mammalian genome : official journal of the International Mammalian Genome Society. 2014;25:211–22. doi: 10.1007/s00335-014-9508-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Logan RW, Robledo RF, Recla JM, Philip VM, Bubier JA, Jay JJ, et al. High-precision genetic mapping of behavioral traits in the diversity outbred mouse population. Genes, brain, and behavior. 2013;12:424–37. doi: 10.1111/gbb.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Svenson KL, Gatti DM, Valdar W, Welsh CE, Cheng R, Chesler EJ, et al. High-resolution genetic mapping using the Mouse Diversity outbred population. Genetics. 2012;190:437–47. doi: 10.1534/genetics.111.132597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tabachnick BG, Fidell LS. Using Multivariate Statistics. 5. Pearson; Boston: 2007. [Google Scholar]
- 22.Johnson JE, Pesek EF, Newland MC. High-rate operant behavior in two mouse strains: a response-bout analysis. Behavioural processes. 2009;81:309–15. doi: 10.1016/j.beproc.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Papachristos EB, Gallistel CR. Autoshaped head poking in the mouse: a quantitative analysis of the learning curve. Journal of the experimental analysis of behavior. 2006;85:293–308. doi: 10.1901/jeab.2006.71-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darvas M, Wunsch AM, Gibbs JT, Palmiter RD. Dopamine dependency for acquisition and performance of Pavlovian conditioned response. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:2764–9. doi: 10.1073/pnas.1400332111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beckmann JS, Bardo MT. Environmental enrichment reduces attribution of incentive salience to a food-associated stimulus. Behavioural brain research. 2012;226:331–4. doi: 10.1016/j.bbr.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson RI, Bush PC, Spear LP. Environmental manipulations alter age differences in attribution of incentive salience to reward-paired cues. Behavioural brain research. 2013;257:83–9. doi: 10.1016/j.bbr.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simon NW, Mendez IA, Setlow B. Effects of prior amphetamine exposure on approach strategy in appetitive Pavlovian conditioning in rats. Psychopharmacology. 2009;202:699–709. doi: 10.1007/s00213-008-1353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lomanowska AM, Lovic V, Rankine MJ, Mooney SJ, Robinson TE, Kraemer GW. Inadequate early social experience increases the incentive salience of reward-related cues in adulthood. Behavioural brain research. 2011;220:91–9. doi: 10.1016/j.bbr.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 29.Anselme P, Robinson MJ, Berridge KC. Reward uncertainty enhances incentive salience attribution as sign-tracking. Behavioural brain research. 2013;238:53–61. doi: 10.1016/j.bbr.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD, et al. Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PloS one. 2012;7:e38987. doi: 10.1371/journal.pone.0038987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DiFeliceantonio AG, Berridge KC. Which cue to ‘want’? Opioid stimulation of central amygdala makes goal-trackers show stronger goal-tracking, just as sign-trackers show stronger sign-tracking. Behavioural brain research. 2012;230:399–408. doi: 10.1016/j.bbr.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flagel SB, Waselus M, Clinton SM, Watson SJ, Akil H. Antecedents and consequences of drug abuse in rats selectively bred for high and low response to novelty. Neuropharmacology. 2014;76(Pt B):425–36. doi: 10.1016/j.neuropharm.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belin D, Deroche-Gamonet V. Responses to novelty and vulnerability to cocaine addiction: contribution of a multi-symptomatic animal model. Cold Spring Harbor perspectives in medicine. 2012;2 doi: 10.1101/cshperspect.a011940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bardo MT, Neisewander JL, Kelly TH. Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacological reviews. 2013;65:255–90. doi: 10.1124/pr.111.005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kliethermes CL, Crabbe JC. Genetic independence of mouse measures of some aspects of novelty seeking. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5018–23. doi: 10.1073/pnas.0509724103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dickson PE, Ndukum J, Wilcox T, Clark J, Roy B, Zhang L, et al. Association of novelty-related behaviors and intravenous cocaine self-administration in Diversity Outbred mice. Psychopharmacology. 2014 doi: 10.1007/s00213-014-3737-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fitzpatrick CJ, Gopalakrishnan S, Cogan ES, Yager LM, Meyer PJ, Lovic V, et al. Variation in the form of Pavlovian conditioned approach behavior among outbred male Sprague-Dawley rats from different vendors and colonies: sign-tracking vs. goal-tracking. PloS one. 2013;8:e75042. doi: 10.1371/journal.pone.0075042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collaborative Cross C. The genome architecture of the Collaborative Cross mouse genetic reference population. Genetics. 2012;190:389–401. doi: 10.1534/genetics.111.132639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts A, Pardo-Manuel de Villena F, Wang W, McMillan L, Threadgill DW. The polymorphism architecture of mouse genetic resources elucidated using genome-wide resequencing data: implications for QTL discovery and systems genetics. Mammalian genome : official journal of the International Mammalian Genome Society. 2007;18:473–81. doi: 10.1007/s00335-007-9045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Genetic association of distance traveled in a novel open field and sign- and goal-tracking in male and female A/J, C57BL/6J, 129S1/SvImJ, NOD/ShiLtJ, and CAST/EiJ inbred mouse strains.