Figure 1. Ca2+ homeostasis in the ER.
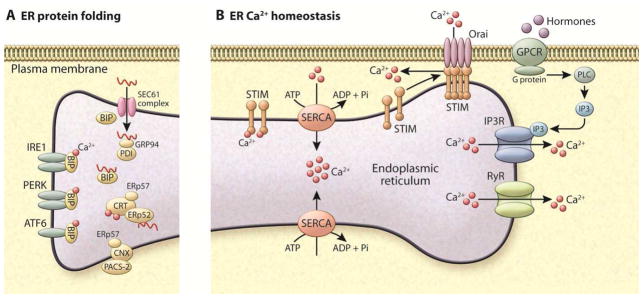
Right: tool-kit of Ca2+ handling proteins. Ligand binding of GPCRs leads to the biosynthesis of the intracellular messengers such as IP3, which diffuses into the cell and binds to IP3R, stimulating Ca2+ release from the ER. Ca2+ channels activated by IP3 release Ca2+ from ER to the cytosol. RyR also releases Ca2+ from ER to the cytosol. SERCA (Ca2+-ATPase) pumps Ca2+ from cytosol to the ER lumen at the expense of ATP hydrolysis. STIM is an ER membrane protein that possesses 2 EF hand domains. Under basal conditions, Ca2+ is bound to these domains and the protein remains in its monomeric form. After Ca2+ release from ER, Ca2+ dissociates from the of STIM proteins leading to oligomerization and trafficking of STIM through the ER membrane toward the plasma membrane contact sites. This localization, allows STIM to couple with the plasma membrane Ca2+ channel Orai and Ca2+ enters the cell from the extracellular space, a process known as capacitive or store operator Ca2+ entry (SOCE). SERCA colocalizes with STIM/Orai complex, and is thought to pump Ca2+ directly from cytosol to ER lumen. Left: ER protein folding machinery: During glycoprotein biosynthesis, the translation of nascent polypeptides is followed by their translocation of the peptides through the SEC61 pore. Sugar molecules are added to the polypeptides that are recognized by lectin chaperones such as calnexin (CNX) and calretculin (CRT), which function in complex with ERp57 and ERp72. Additional chaperone assistance is provided by the ATP-driven chaperone BiP (GRP78). Calnexin and calreticulin also bind Ca2+ with high capacity, functioning as the main Ca2+ buffers in the ER lumen. Ca2+ depletion leads to accumulation of misfolded proteins and the activation of the unfolded protein response (UPR), mediated by three canonical ER luminal sensors, IRE1, PERK, and ATF6, which are inactive and bound to BiP in the absence of stress. GPCR: G protein–coupled receptor. IP3: inositol triphosphate. RyR: Ryanodine receptor. STIM1: stroma interacting protein.
