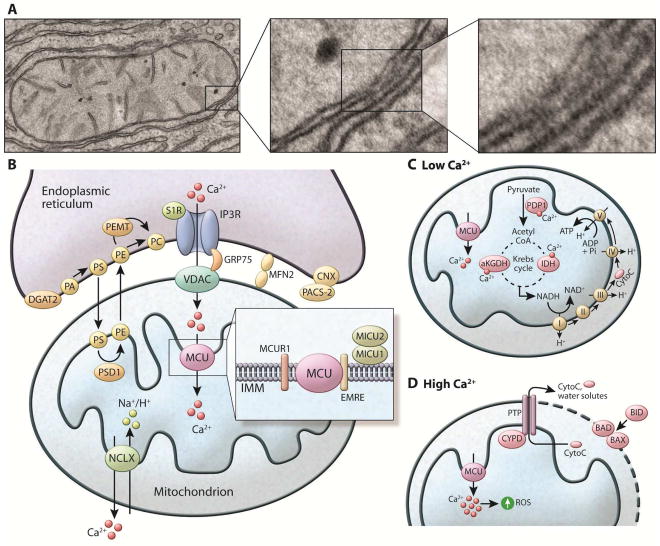
Figure 2. Components of the mitochondria associated ER membranes (MAMs) and Ca2+ homeostasis in the mitochondria (A).
Electron microscopic images exemplifying the close contacts between smooth ER and mitochondria in mouse liver cells. Dense areas show the proteinaceous bridges formed between the two membranes. (B) The MAM connection is mediated by protein tethers such as mitofusin2 (MFN2) and stabilized by proteins such as PACS-2 bound to ER-resident calnexin (CNX). The MAMs are enriched in proteins that regulate Ca2+ transport from ER to the mitochondria such as IP3R, connected to the mitochondria anion transporter (VDAC) by the chaperone GRP75. Through these structures Ca2+ leaves the ER and enters the mitochondria matrix via the mitochondria Ca2+ uniporter (MCU) located in the IMM. MCU is regulated by a series of binding partners such as MICU1 and MICU2, MICUR1 and EMRE. MICU1 functions as a gatekeeper for MCU-mediated Ca2+ uptake. MICU2 seems to inhibit MCU function. EMRE is essential for the interaction between MCU and the MICU proteins and is indispensable for MCU-mediated Ca2+ transport. The exit pathway for mitochondrial Ca2+ is mediated by the NCXL antiporter that exchanges Na+/Ca2+. MAMs are also enriched in key enzymes of lipid biosynthesis to facilitate the conversion of PA to PE and PC, as described in the text. (C) Within the mitochondria, Ca2+ regulates the activity of matrix and citric acid cycle enzymes such as pyruvate dehydrogenase phosphatase (PDP1), α-ketoglutarate dehydrogenase (aKGDH), and isocitrate dehydrogenase (IDH). This culminates in increased NADH production that feeds the respiratory chain to produce ATP. (D) Mitochondrial Ca2+ overload leads to increased mitochondrial ROS production and the opening the mitochondrial permeability transition pore (PTP). The opening of the PTP leads to a collapse of membrane potential and mitochondrial swelling, with consequent loss of nucleotides and cytochrome C (CytoC), and is directly linked with apoptotic cell death. Apoptosis is regulated by the BCL2 class of proteins such as BID, BAX and BAD. Under normal conditions, the proapoptotic protein BAX is in the cytosol. However, under apoptotic condition this protein interacts with BAD or BID located in the OMM, stimulating the permeabilization of the OMM.