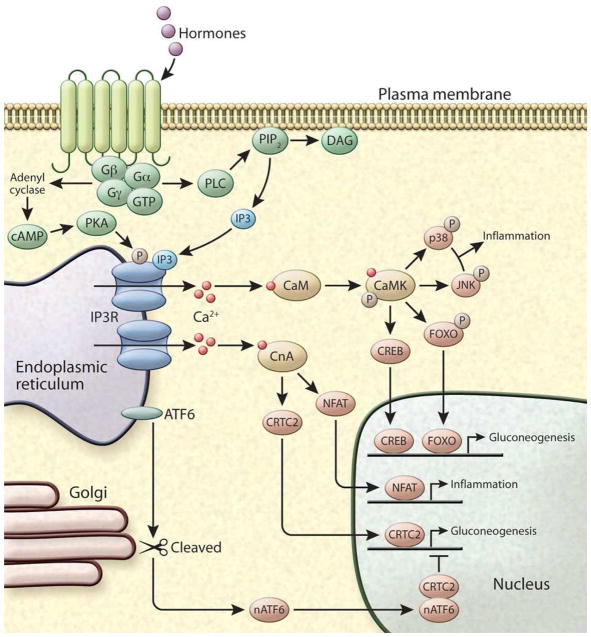
Figure 3. Intracellular Ca2+ signaling.
GPCR activation by hormones can induce Ca2+ release by activating phospholipase C (PLC) leading to the hydrolysis of phosphatidylinositol 4,5 bisphosphate (PIP2) to produce the intracellular messengers IP3 and DAG. IP3 diffuses in to the cell and binds to IP3R, stimulating Ca2+ release from the ER. Alternatively, GPCR activation leads to the production of cAMP, which activates the protein kinase A (PKA), enabling it to phosphorylate IP3R and induce its activity. In the cytosol the two main Ca2+ sensors involved in signal transduction are CaM kinase (CamK) and Calcineurin (CLN). CaMKs have been shown to directly phosphorylate many targets including the inflammatory proteins JNK and p38 and transcription factors including FOXO and CREB. CLN is activated by sustained high Ca2+ levels and regulates the activity of at least three important transcription pathways involving Nuclear factor of activated T-cells (NFAT) and CREB-regulated transcription coactivator 2 (CRTC2).