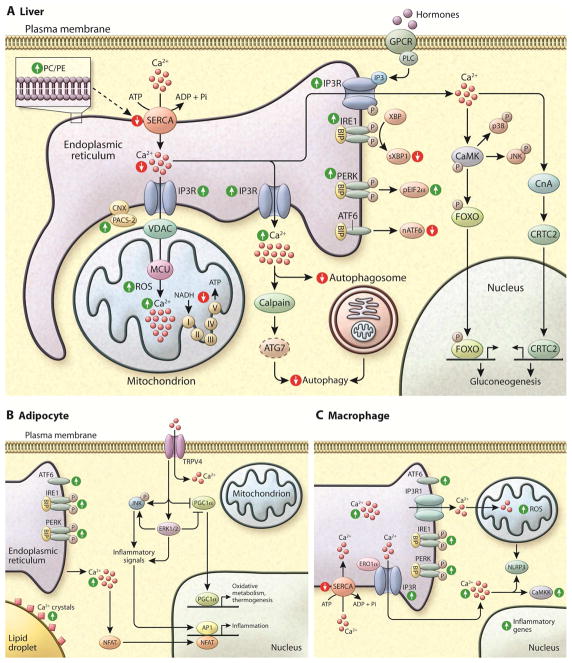
Figure 4. Dysfunction of Ca2+ homeostasis in metabolic disease.
(A) In the liver of obese humans and animal models, the UPR is activated and the phosphorylation of IRE1 (inositol required 1), PERK (PKR-like endoplasmic reticulum localized kinase), and eIF2α are increased. However, the levels of sXBP1 and ATF6 (activating transcription factor 6) activity are decreased in obesity in the liver (similar to beta cells). Due to changes in ER membrane composition (increased ratio of PC/PE) the transport of Ca2+ from cytosol to ER lumen by SERCA is impaired, leading to decreased Ca2+ levels in ER. Also, in the liver of obese animals IP3R1 expression and activity are increased, which contributes both to decreased levels of ER Ca2+ and increased cytosolic and mitochondrial Ca2+. In the mitochondria, Ca2+ overload correlates with increased oxidative stress (ROS) and decreased oxidative function. Increased cytosolic Ca2+ also affects several cellular pathways such as autophagosome accumulation, and activates proteases such as calpain that cleaves and degrades ATG7. Both phenomena culminate in decreased autophagy, as seen in liver in obesity. Increased cytosolic Ca2+ also activates calcineurin leading to dephosphorylation of CRTC2 and its nuclear translocation, and activates CaMK to phosphorylate FOXO, with a direct impact on gluconeogenesis. CaMK also phosphorylates p38 and JNK leading to increased inflammation.
In both adipocytes (B) and macrophages (C) of obese animal models, the UPR is activated and the phosphorylation of IRE1, PERK, ATF6 and eIF2α are increased. (B) In hypertrophic adipocytes there is increased deposition of Ca2+ surrounding the lipid droplets and increased cytosolic Ca2+ levels. NFAT transcription factors isoforms 2 and 4 are induced in obese adipose tissue driving expression of inflammatory genes. TRPV4 (Transient receptor potential cation channel subfamily V member 4) is a Ca2+-permeable ion channel that was first identified as an osmolality sensor. In adipocytes, it acts as a negative regulator of PGC1α and a positive regulator of inflammatory genes and proteins and JNK activity. These effects are related to the activation of ERK1/2. (C) Macrophages derived from obese animals show increased IP3R1 and ERO1α activity, which leads to higher Ca2+ release from ER to cytosol. In addition reduced levels of SERCA2b mRNA and protein expression is observed in this condition. Rise in cytosolic Ca2+ and mitochondrial oxidative stress have been shown to engage the NLRP3 inflammasome pathway. In addition, the alterations in macrophage activity in obesity have been shown to activate CaMKK (Calcium/calmodulin-dependent protein kinase kinase 2), the deletion of which leads to impaired cytokine secretion and phagocytosis and induces morphological changes.