Figure 2.
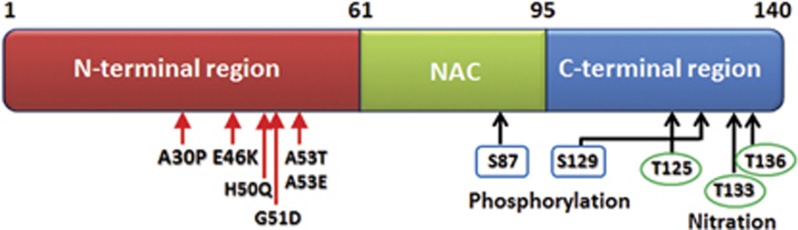
Structure of full length α-SYN protein and its functional components. N-terminal (1–60), non-Aβ component of amyloid plaques domain (61–95) and C-terminal (96–140); point mutations reported in familial PD (autosomal dominant form) are present in the N-terminal (red arrows). N-terminal contributes to the α-helical structure of α-SYN upon binding to lipid membranes. Non-Aβ component of amyloid plaques domain contains most hydrophobic residues and one phosphorylation site (blue rectangle, serine 87). Non-Aβ component of amyloid plaques domain promotes aggregation of the molecule by its propensity to form β-sheet structure. C-terminal has three nitration sites (green circle, tyrosine 125, 133, 136) and one phosphorylation site (blue rectangle, serine S129) and is mainly responsible for maintaining the protein solubility.
