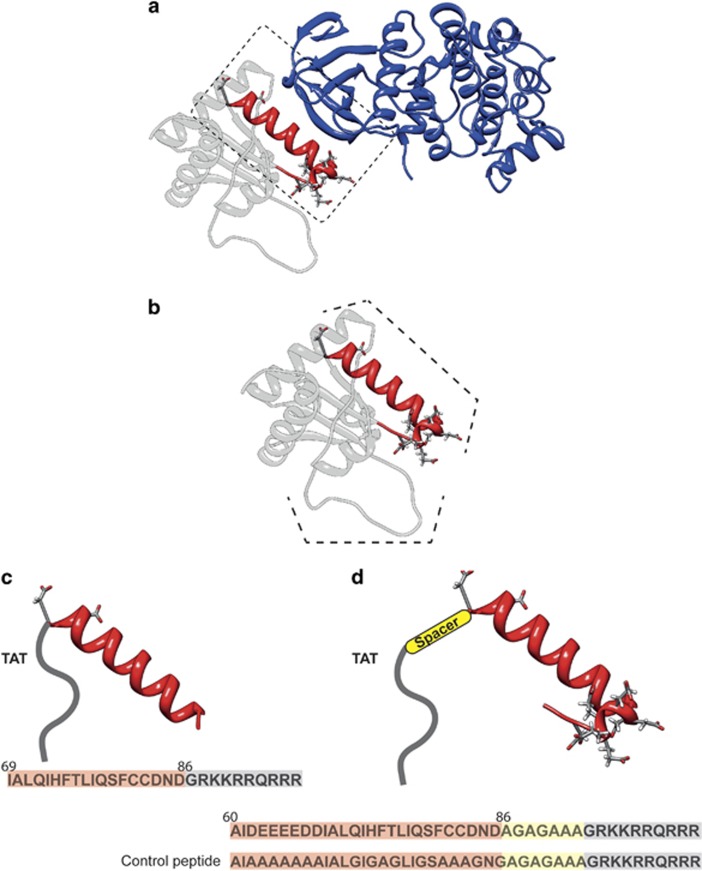
Figure 1.
Design of specific inhibitors of MKK7 based on GADD45β structure. (a) Ribbon representation of the best complex resulting from the docking between the modeled structure of GADD45β and MKK7. MKK7 is shown in blue and GADD45β in gray. The helix-turn motif of GADD45β involved in the interaction, taken as a template to design the peptides, has been reported in red. (b) Structure of GADD45β protein obtained by homology modeling. Residues 60–86 forming a helix-turn motif are highlighted in red and with a dashed line. Residues 104–113 form a long loop with an alternation of hydrophilic and negative residues, this region is highlighted with a dashed line. (c) Modeling of TAT-GADD45β69–86 peptide. In red is the sequence of the α-helix (residues 69–86) and in gray the sequence of TAT. The entire sequence of the peptide is shown on the bottom. (d) Modeling of TAT-spacer-GADD45β60–86 peptide. In red is showed the sequence of the the α-helix (residues 60–86), in yellow the position of the linker and in gray the TAT sequence. The entire sequence of the peptide is shown on the bottom. The sequence of the TAT-GADD45β69–86 CONTROL is also reported