Abstract
In dialyzed patients, preservation of residual renal function is associated with better survival, lower morbidity, and greater quality of life. To analyze the evolution of residual diuresis over time, we prospectively monitored urine output in 401 pediatric patients in the global IPPN registry who commenced peritoneal dialysis (PD) with significant residual renal function. Associations of patient characteristics and time-variant covariates with daily urine output and the risk of developing oligoanuria (under 100 ml/m2/day) were analyzed by mixed linear modeling and Cox regression analysis including time-varying covariates. With an average loss of daily urine volume of 130 ml/m2 per year, median time to oligoanuria was 48 months. Residual diuresis significantly subsided more rapidly in children with glomerulopathies, lower diuresis at start of PD, high ultrafiltration volume, and icodextrin use. Administration of diuretics significantly reduced oligoanuria risk, whereas the prescription of renin–angiotensin system antagonists significantly increased the risk oligoanuria. Urine output on PD was significantly associated in a negative manner with glomerulopathies (−584 ml/m2) and marginally with the use of icodextrin (−179 ml/m2) but positively associated with the use of biocompatible PD fluid (+111 ml/m2). Children in both Asia and North America had consistently lower urine output compared with those in Europe perhaps due to regional variances in therapy. Thus, in children undergoing PD, residual renal function depends strongly on the cause of underlying kidney disease and may be modifiable by diuretic therapy, peritoneal ultrafiltration, and choice of PD fluid.
Keywords: children, oligoanuria, peritoneal dialysis, registry, risk factors, urine volume
Chronic dialysis is associated with high patient morbidity and mortality. Outcome studies suggest that residual renal function (RRF) is a more important determinant of patient survival, morbidity, and quality of life than the prescribed or achieved dialysis dose.1, 2, 3 As RRF is generally considered a largely unmodifiable and rapidly diminishing fraction of fluid and solute clearance in dialyzed patients, a limited body of research has explored its determinants and amenability to therapeutic intervention. These studies have suggested a major impact of the underlying renal disease,4, 5, 6, 7, 8 baseline RRF5, 9, and dialysis modality,9, 10, 11, 12 with possible additional effects of ethnicity,4 gender,4, 13 obesity,13, 14, 15 medications,4, 14, 16, 17 infections,13, 18 cardiovascular events,19 and dialysis biocompatibility.20
RRF appears to be particularly important in dialyzed children, where it has been associated with better nutritional status and growth, cardiovascular function, and survival.21, 22, 23, 24 Congenital anomalies of kidney and urinary tract (CAKUT) are the leading cause of end-stage renal disease (ESRD) in children, and these disorders are typically characterized by the preservation of high urine output even in CKD stage V. In addition, peritoneal dialysis (PD), which tends to preserve RRF better than hemodialysis,4, 9, 25, 26, 27 is the dialysis modality of first choice in children.
Although the pediatric chronic peritoneal dialysis (CPD) population might be particularly well suited to study underlying conditions and therapeutic measures influencing RRF, the low incidence of pediatric ESRD has so far precluded large prospective studies assessing the course of RRF in children commencing chronic dialysis. To overcome this challenge, we interrogated the global IPPN Registry, a comprehensive prospective database encompassing more than 2000 pediatric patients undergoing CPD in 40 countries around the globe.28 We identified 401 children who commenced CPD with significant RRF and analyzed the factors associated with the evolution of residual diuresis over time.
RESULTS
Cohort selection and patient characteristics
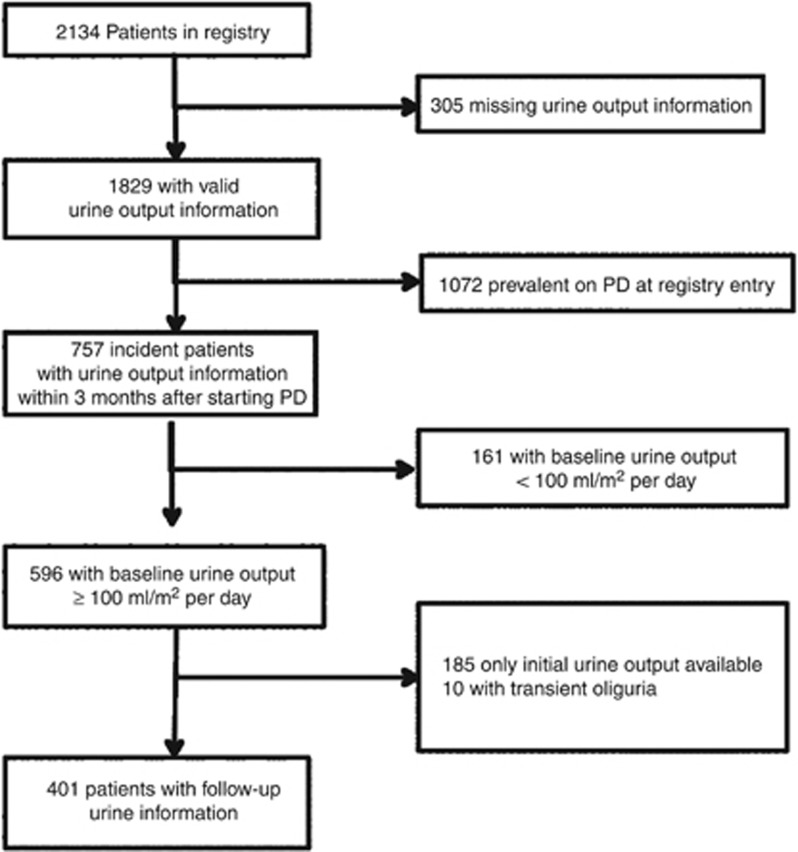
The selection of the study cohort from the IPPN registry population is described in Figure 1. Between April 2007 and June 2013, 2134 pediatric CPD patients were enrolled at 100 pediatric dialysis centers in 39 countries. Among 1829 patients with valid information on daily urine volume, 757 patients were incident with diuresis information reported within 3 months of CPD initiation. Of these, 161 patients were excluded because of oligoanuria (defined by daily urine output ≤100 ml/m2 body surface area) at the start of CPD. Of the remaining 596 patients, 185 children with only a single urine volume measurement and 10 patients in whom urine output decreased transiently were excluded, leaving 401 children for analysis. The cohort was largely representative of the total IPPN cohort and the pediatric PD population, as reported to population-based registries in Europe and the United States (Supplementary Table S-1 online).
Figure 1.
Selection of study subjects. PD, peritoneal dialysis.
The characteristics of the study cohort are given in Table 1 and Supplementary Table S-2 online. The median duration of follow-up was 17 (IQR: 10–27) months. Among the 401 patients, 299 exhibited preserved diuresis during follow-up, whereas 102 developed oligoanuria. The most common primary renal diagnosis was CAKUT (50%), followed by glomerulopathies (30%). Children with CAKUT had higher urine output at PD start than patients with glomerulopathies (1.26±0.71 vs. 0.77±0.52 l/m2/day), whereas eGFR tended to be lower in CAKUT patients (9.0±4.5 vs.10.2±5.5 ml/min/1.73 m2).
Table 1. Patient characteristics, stratified according to prospective evolution of diuresis.
| All | Preserved diuresis | Oligoanuria during follow-up | P-value | |
|---|---|---|---|---|
| N | 401 | 299 | 102 | |
| Total observation time (years) | 1.26 (1.46) | 1.10 (1.42) | 1.55 (1.49) | 0.001 |
| Male sex | 218 (54.4%) | 171 (57.2%) | 47 (46.1%) | 0.052 |
| Age (years) | 9.6 (10.6) | 9.9 (10.6) | 8.6 (10.4) | 0.285 |
| Pubertal | 162 (40.4%) | 126 (42.1%) | 36 (35.3%) | 0.224 |
| Ethnicity | ||||
| Caucasian | 246 (61.3%) | 186 (62.2%) | 60 (58.8%) | 0.545 |
| Other | 155 (38.7%) | 113 (37.8%) | 42 (41.2%) | |
| Gross national income (1,000 Intern'l $) | 23.0±11.4 | 22.6±10.8 | 24.2±12.8 | 0.216 |
| Underlying diagnosis | ||||
| CAKUT | 200 (49.9%) | 175 (58.5%) | 25 (24.5%) | <0.001 |
| Glomerulopathies | 122 (30.4%) | 67 (22.4%) | 55 (53.9%) | |
| Other | 79 (19.7%) | 57 (19.1%) | 22 (21.6%) | |
| BMI s.d. scores | −0.26±1.43 | −0.23±1.45 | −0.36±1.37 | 0.422 |
| Δ Height s.d. scores per year | −0.03±0.96 | 0.01±0.90 | −0.16±1.08 | 0.106 |
| Estimated fluid excess (%) | 1.34±2.43 | 1.07±2.01 | 2.12±3.26 | 0.003 |
| Blood pressure s.d. scores | ||||
| Systolic | 1.02±1.39 | 0.85±1.28 | 1.51±1.56 | <0.001 |
| Diastolic | 0.92±1.33 | 0.84±1.22 | 1.17±1.59 | 0.063 |
| Medications | ||||
| ≥2 Antihypertensive drugs | 125 (31.2%) | 76 (25.4%) | 49 (48.0%) | <0.001 |
| RAS antagonist | 129 (39.2%) | 82 (33.5%) | 47 (56.0%) | <0.001 |
| Diuretics | 85 (25.8%) | 63 (25.7%) | 22 (26.2%) | 0.931 |
| Urine output (l/m2 per day) | 1.00±0.63 | 1.11±0.69 | 0.67±0.48 | <0.001 |
| Urinary GFR (ml/min/1.73m2)a | 5.2±5.0 | 5.9±5.2 | 2.8±3.2 | <0.001 |
| Weekly Kt/V ureaa | ||||
| Urinary | 1.55±2.13 | 1.77±2.31 | 0.84±1.13 | 0.004 |
| Dialytic | 1.97±2.28 | 1.89±2.56 | 2.22±0.96 | 0.320 |
| Total | 3.48±4.02 | 3.63±4.54 | 3.01±1.45 | 0.294 |
| PD modality | ||||
| CAPD | 97 (24.2%) | 71 (23.7%) | 26 (25.5%) | 0.375 |
| NIPD (APD with dry day) | 188 (46.9%) | 146 (48.8%) | 42 (41.2%) | |
| CCPD (APD with wet day) | 116 (28.9%) | 82 (27.4%) | 34 (33.3%) | |
| PD fluids | ||||
| Biocompatible PD fluid | 171 (42.6%) | 125 (41.8%) | 46 (45.1%) | 0.562 |
| Icodextrin | 32 (8.0%) | 18 (6.0%) | 14 (13.7%) | 0.013 |
| Glucose exposure (g/kg per day) | 3.56±2.07 | 3.37±1.99 | 4.11±2.20 | 0.002 |
| Total PD fluid turnover(l/m2 per day) | 5.53±2.67 | 5.43±2.65 | 5.83±2.72 | 0.199 |
| Ultrafiltration volume (l/m2 per day) | 0.43±0.33 | 0.38±0.29 | 0.59±0.39 | <0.001 |
| Patients with >1 peritonitis | 142 (35.4%) | 101 (33.8%) | 41 (40.2%) | 0.242 |
| Exposure to nephrotoxic drugs (days) | 0.8±4.5 | 1.0±5.4 | 0.2±1.5 | 0.428 |
Abbreviations: APD, automated peritoneal dialysis; BMI, body mass index; CAKUT, congenital anomalies of kidney and urinary tract; CAPD, continuous ambulatory peritoneal dialysis; CCPD, continuous cycling peritoneal dialysis; GFR, glomerular filtration rate; NIPD, nocturnal intermittent peritoneal dialysis; PD, peritoneal dialysis; RAS, renin–angiotensin system. Data are given as N (%), mean±s.d., or median (interquartile range). P values denote significant differences between patients with retained diuresis and those progressing to oligoanuria.
Measured in 180 patients.
In 180 of the 401 patients, the results of 603 twenty-four-hour urine and dialysate collections were available for analysis. Information on the peritoneal transport status obtained from Peritoneal Equilibration Tests was available in 200 subjects.
Determinants of residual diuresis
The univariate exploration of factors potentially associated with progressive loss of residual diuresis is given in Table 1. Relative to the children who became oligoanuric during the observation period, children who retained diuresis frequently had CAKUT as underlying renal disease, exhibited a larger urine output at the time of PD initiation, were exposed to lower dialysate glucose and less frequently to icodextrin, and achieved lower daily ultrafiltration rates (Table 1). They showed a lower degree of estimated fluid excess, lower blood pressure, and were administered less antihypertensive agents including renin–angiotensin system (RAS) antagonists. In contrast, patients with stable versus vanishing diuresis did not differ by age, ethnicity, body mass index, PD treatment modality, total PD fluid turnover, dialytic clearance, the use of biocompatible PD fluid or of diuretics, peritonitis frequency, and the cumulative exposure to nephrotoxic drugs (aminoglycosides and glycopeptides). The duration of follow-up was longer in patients who became oligoanuric than in those with stable diuresis (20.8 (IQR: 12.8–31.8) vs. 15.4 (8.8–25.5) months, P=0.001).
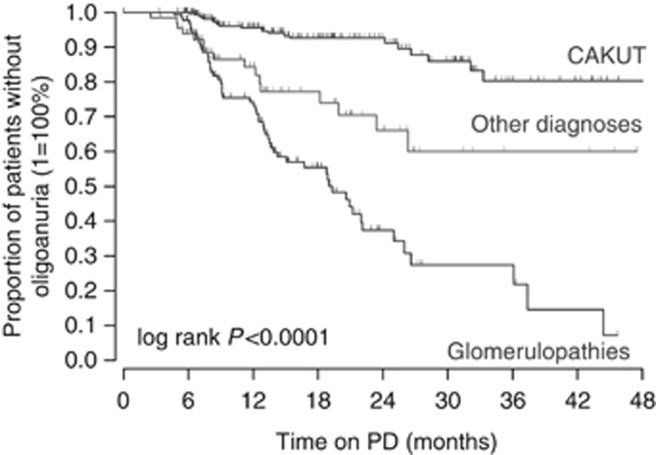
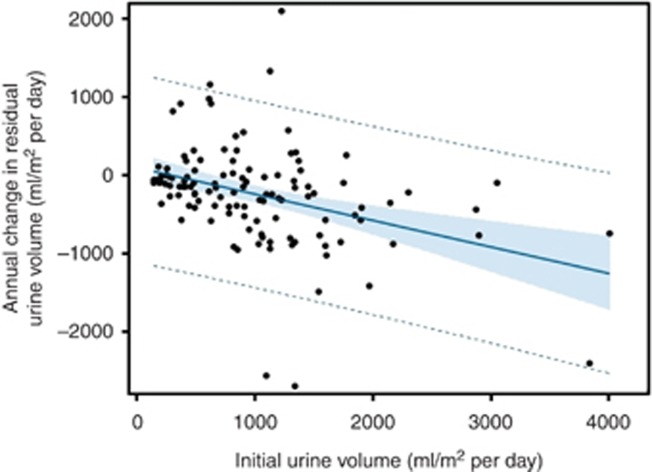
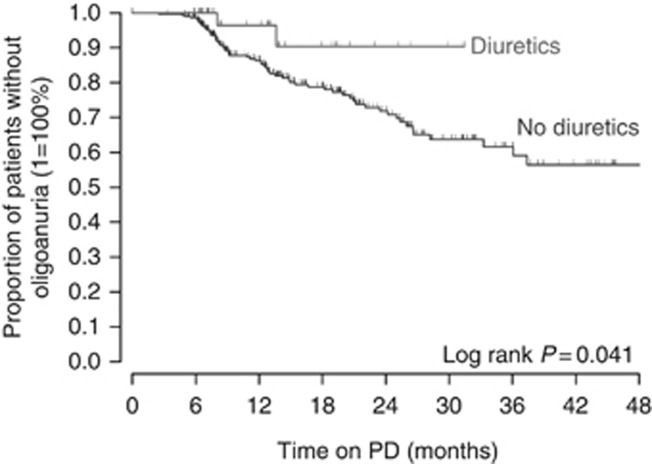
Cox regression analysis was performed to identify independent risk factors of progression to oligoanuria, including time-variant covariates (Table 2). This analysis confirmed an almost fourfold higher risk of oligoanuria in patients with glomerulopathic disorders relative to those with CAKUT (P<0.0001). The corresponding Kaplan–Meier survival analysis of residual diuresis revealed significantly earlier loss of urine volume in patients with glomerulopathies (P<0.0001, Figure 2). It was also demonstrated that a high initial residual urine volume lowers oligoanuria risk independently of the kidney disease type (P<0.0001), although the annual loss of daily urine output was slightly more pronounced in patients with higher initial urine output (Figure 3). Interestingly, initial urine output was not replaceable by eGFR in predicting the oligoanuria risk. In addition, the use of icodextrin (P=0.001) and higher achieved ultrafiltration rates (P=0.002) were significant risk factors of oligoanuria. After adjusting for the underlying disease type, baseline urine output, and dialytic fluid removal, treatment with diuretics was associated with an 82% oligoanuria risk reduction during the observation period (P=0.004). The diuresis survival curves of children with and without diuretic therapy from the start of PD are shown in Figure 4. Conversely, the use of RAS antagonists tended to increase the risk of becoming oligoanuric (P=0.04). Body mass index, PD modality, and the number of peritonitis episodes did not affect the oligoanuria risk. The observed associations were independent of the region of residence.
Table 2. Extended Cox regression analysis of factors predicting risk of developing oligoanuria.
|
Full model |
Reduced model |
|||||
|---|---|---|---|---|---|---|
| HR | (95% CI) | P-value | HR | (95% CI) | P-value | |
| Male sex | 0.961 | (0.616–1.498) | 0.861 | |||
| Age at initiation of PD (year) | 0.953 | (0.899–1.011) | 0.112 | 0.953 | (0.914–0.992) | 0.026 |
| Puberty | 0.918 | (0.503–1.654) | 0.777 | |||
| BMI s.d. scores | 1.148 | (0.952–1.391) | 0.153 | 1.154 | (0.965–1.386) | 0.121 |
| Estimated fluid excess (%) | 1.102 | (1.006–1.200) | 0.030 | 1.080 | (0.993–1.163) | 0.056 |
| Systolic blood pressure s.d. scores | 0.963 | (0.834–1.109) | 0.606 | |||
| Underlying diagnosis (reference: CAKUT) | ||||||
| Glomerulopathies | 4.134 | (2.339–7.527) | <.0001 | 4.776 | (2.791–8.467) | <0.0001 |
| Other | 2.160 | (1.012–4.541) | 0.043 | 2.607 | (1.272–5.238) | 0.015 |
| Initial urine output (l/m2 per day) | 0.470 | (0.285–0.743) | 0.002 | 0.441 | (0.278–0.672) | <0.0001 |
| Medications | ||||||
| RAS antagonists | 1.603 | (0.979–2.631) | 0.061 | 1.546 | (1.018–2.346) | 0.040 |
| Diuretics | 0.170 | (0.041–0.475) | 0.003 | 0.178 | (0.043–0.486) | 0.004 |
| PD modality (reference: CAPD) | ||||||
| NIPD | 0.974 | (0.422–2.406) | 0.952 | |||
| CCPD | 0.861 | (0.407–1.990) | 0.714 | |||
| PD fluids | ||||||
| Biocompatible PD fluid | 0.881 | (0.453–1.716) | 0.710 | |||
| Icodextrin | 2.380 | (1.327–4.196) | 0.003 | 2.285 | (1.364–3.699) | 0.001 |
| Ultrafiltration volume (l/m2 per day) | 1.811 | (1.328–2.462) | <.0001 | 1.885 | (1.253–2.120) | <0.0001 |
| No. of peritonitis episodes | 0.992 | (0.790–1.205) | 0.937 | |||
| Nephrotoxic drug exposure (days) | 0.905 | (0.751–1.067) | 0.263 | |||
| Region of residence (reference: Europe) | ||||||
| United States | 0.928 | (0.279–2.623) | 0.895 | |||
| Latin America | 0.563 | (0.230–1.349) | 0.201 | |||
| Turkey | 0.905 | (0.751–1.067) | 0.263 | |||
| Asia | 1.050 | (0.473–2.280) | 0.090 | |||
Abbreviations: BMI, body mass index; CAKUT, congenital anomalies of kidney and urinary tract; CAPD, continuous ambulatory peritoneal dialysis; CCPD, continuous cycling peritoneal dialysis; CI, confidence interval; GFR, glomerular filtration rate; HR, hazard ratio; PD, peritoneal dialysis; NIPD, nocturnal intermittent peritoneal dialysis; RAS, renin–angiotensin system.
Figure 2.
Survival of residual diuresis by the renal diagnosis group.
Figure 3.
Association of initial urine output and the subsequent annualized loss of residual diuresis (R2=0.1389, P <0.0001).
Figure 4.
Survival of residual diuresis in 45 patients receiving diuretic therapy compared with patients without diuretics.
A separate proportionate hazard analysis was performed for the subgroup of patients with available Peritoneal Equilibration Test information. The hazard ratio to turn oligoanuric did not differ between patients with low, low-average, high-average, and high transporter status (overall P=0.487).
In addition to the extended Cox regression analysis of the oligoanuria risk performed on the entire cohort, we utilized mixed linear modeling to identify factors predicting residual urine volume at any time on PD as a continuous variable in those 180 patients in whom precise urine volume measurements from 24-h urine collections were available (Table 3). All factors significantly associated with the development of oligoanuria (Table 1) were offered for inclusion in the model. A strong linear trend toward lower diuresis with time on dialysis was observed (P<0.0001); a mean annual loss of 138 (95% confidence interval: 92 to 184) ml/m2 residual urine volume was calculated by univariate regression for the population as a whole (Table 3, Model 1). Independently of time on dialysis, the diagnosis of glomerulopathy or other non-CAKUT disease and the use of icodextrin were associated with lower urine output, whereas the use of biocompatible PD fluids and, at borderline significance, diuretics positively predicted residual urine volume. As some factors such as PD duration and the use of biocompatible PD fluid, icodextrin, and diuretics strongly differed by region (see Supplementary Table S-1 online), another model including the region of residence was calculated (Table 3, Model 2). This analysis disclosed that patients in the United States, Turkey, and Asia had consistently lower urine output than children treated in Europe. The inclusion of region variably attenuated the effect of diuretics, biocompatible PD fluid, and icodextrin.
Table 3. Mixed linear model analysis of factors predicting residual urine volume.
| Variables | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| Estimate | s.e. | P-value | Estimate | s.e. | P-value | |
| Intercept | 1125 | 104 | <0.0001 | 1350 | 106 | <0.0001 |
| Time on PD (years) | −138 | 23 | <.0001 | −134 | 23.4 | <0.0001 |
| Underlying diagnosis (ref. CAKUT) | ||||||
| Glomerulopathies | −614 | 103 | <0.0001 | −584 | 101 | <0.0001 |
| Other | −395 | 145 | 0.007 | −330 | 142 | 0.021 |
| Use of diuretics | 103 | 59 | 0.074 | 84 | 61 | 0.165 |
| Use of biocompatible PD fluid | 121 | 59 | 0.028 | 111 | 58 | 0.057 |
| Use of icodextrin | −202 | 100 | 0.043 | −179 | 103 | 0.083 |
| Ultrafiltration volume (l/m2 per day) | −35 | 23 | 0.138 | −42 | 24 | 0.077 |
| Region of residence (ref: Europe) | ||||||
| North America | −431 | 215 | 0.047 | |||
| Latin America | 46 | 120 | 0.705 | |||
| Turkey | −259 | 131 | 0.050 | |||
| Asia | −420 | 153 | 0.007 | |||
Abbreviations: CAKUT, congenital anomalies of kidney and urinary tract; PD, peritoneal dialysis. Daily urine output per m2 body surface area was used as the dependent variable in the model. Estimates denote ml/m2 diuresis difference attributable to predictor variable (per one unit for quantitative variables).
DISCUSSION
Our prospective study in a large cohort of children commencing CPD defines key determinants of residual diuresis in the pediatric ESRD population. Among more than 750 children starting PD, 80% still had significant urine output. For these, the median time to oligoanuria while on PD was 4 years. Hence, although RRF declines with time on dialysis, a large proportion of pediatric PD patients enjoy extended preservation of urine output.
The most important cause for the relatively slow overall loss of residual diuresis was the high proportion (50%) of children with underlying CAKUT. Children with these disorders were four times less likely to develop oligoanuria and had on average >600 ml/m2 higher daily urine output than children with ESRD due to glomerulopathies at any time during the observation period. Although we cannot completely exclude the possibility of overestimated hazard ratios due to informative censoring (e.g. renal transplantation being a competing event), our findings are in keeping with previous reports of glomerulopathies being a risk factor for rapid loss of urine output in adult populations.4, 5, 6, 7, 8 Whereas in adult patients diabetic nephropathy is by far the most prevalent glomerular disease,4, 7, 8 hereditary podocyte disorders are the most common type of glomerulopathy in children. Our findings indicate that these disorders are prone to rapid loss of RRF in a manner similar to what occurs with the acquired glomerular diseases causing ESRD in adults.
We confirmed previous reports that high urine volume at initiation of PD is predictive of sustained diuresis.9 It is noteworthy that this effect was independent of the underlying kidney disease type, suggesting that CAKUT patients maintain diuresis on dialysis intrinsically better than children with other kidney disorders. The effect of disease type was also independent of blood pressure, which, as expected, was higher in the glomerulopathic patients.
It is also of note that patients with CAKUT did not have better eGFR at the start of PD, and the lower oligoanuria risk attributable to high urine output was not replaceable by eGFR. Hence, the advantage of CAKUT patients is limited to the preservation of urine output rather than small-solute clearance.
A notable finding of this study is the positive impact of diuretics on maintaining residual urine output. Children who received diuretics from the initiation of PD were 80% less likely to become oligoanuric than untreated patients. Our findings represent the first evidence suggesting effective stimulation of residual diuresis by diuretics in dialyzed children. The findings of this observational study are in keeping with the results of a randomized controlled trial in adults on CPD in which furosemide increased urine volume without affecting creatinine and urea clearance;29 in contrast, another adult study observed a faster decline of residual GFR associated with diuretic usage.30 The efficacy of diuretics is also controversial in the adult hemodialysis population.16, 31 In view of the crucial role of fluid balance in preventing cardiovascular morbidity in the dialysis population, our findings should stimulate interventional studies to further evaluate the efficacy of diuretic therapy in pediatric and adult dialysis patients.
The observed associations of higher daily ultrafiltration rates and icodextrin use with lower urine volumes and a higher risk of oligoanuria may reflect the efforts for enhanced dialytic fluid removal in patients with failing RRF. However, the observational nature of our study does not allow us to exclude the alternative interpretation of a causative detrimental impact of higher ultrafiltration on residual diuresis. Of interest in this context, in a randomized controlled trial in adult PD patients, the use of icodextrin was associated with a statistically insignificant but slightly more rapid loss of RRF over time.32 In contrast, a recent systematic review concluded that icodextrin improves peritoneal ultrafiltration without compromising RRF or urine output.33
Several randomized clinical trials in adult PD patients evaluated a potential effect of the use of biocompatible PD fluids with low glucose degradation product content on the preservation of RRF.34, 35, 36, 37, 38 Most studies reported better preserved urine output with low glucose degradation product solutions, with a somewhat less consistent effect on small molecule clearance. A recent meta-analysis of seven randomized clinical trials encompassing 520 adult patients identified a mean difference of 126 (95% CI 27–226) ml urine volume per day in favor of biocompatible PD fluids.20 Our multivariate analysis of 180 children attributed a marginal increase of daily urine volume (111±58 ml/m2) to the use of biocompatible PD fluids, although the risk of developing oligoanuria was not reduced.
Blood pressure is a well-established risk factor for the loss of renal function in pre-dialytic CKD both in adults and children, as well as in incident adult dialysis patients.39, 40 In this pediatric PD population, blood pressure was not predictive of residual urine volume or the risk of developing oligoanuria. Although the KDIGO clinical practice guideline recommends RAS inhibition in CKD, few previous studies addressed a potential effect of RAS antagonist therapy on RRF. In 1032 incident adult PD patients followed in a Canadian national registry, the use of ACE inhibitors was associated with a reduced risk of RRF loss defined by urine output <200 ml per day.4 Smaller single-center studies yielded conflicting results regarding the impact of RAS blockade on RRF.8, 17 In our pediatric study, RAS blockade did not appear to preserve overall residual urine volume but marginally increased the risk of developing oligoanuria. We speculate that the pediatric PD population may be at greater risk of episodic volume depletion compared with adults, due to a higher incidence of gastrointestinal and febrile infections in childhood adding to increased urinary fluid and electrolyte losses characteristically present in patients with dysplastic kidney disease. In this setting, RAS blocker therapy might increase the risk of episodic renal hypoperfusion leading to irreversible oligoanuria.
In the same context, studies performed in the 1990s observed that frequent peritonitis episodes predisposed to a rapid loss of RRF.18 We did not observe an association of the number of peritonitis episodes or the duration of exposure to nephrotoxic antibiotics with the evolution of residual diuresis. The markedly decreased overall incidence, earlier detection, and more efficient treatment of peritonitis accomplished in recent years may have largely eliminated the impact of PD-associated infections on RRF.
The estimated deviation of actual body weight from ‘dry' weight did not predict urine volume over time and tended to be associated with an increased oligoanuria risk. This finding does not support the widespread notion that urine output increases with fluid overload.
Other risk factors for RRF loss identified previously in adult patients, i.e. male sex and obesity, were also not found to be significant in our study. This may be explained by the limited number of sexually mature individuals and the low prevalence of obesity in this global pediatric population.
A potential limitation of this study concerns the generalizability of our findings. As reporting to the IPPN Registry is voluntary, we cannot entirely exclude selection bias related to the type of centers reporting to the registry, the patients reported by a particular center, and the availability of urine output information. However, we found the study cohort to be largely representative of the entire IPPN cohort and other international pediatric RRT registry populations regarding the distribution of age and eGFR at the start of PD, as well as the underlying renal disease spectrum (Supplementary Table S-1 online). Likewise, the average follow-up time on PD was comparable to that of the total incident patient population and, at least for the European countries including Turkey, matched those recently reported by the population-based ESPN/ERA-EDTA Registry.41
Global data collection is both a strength and a limitation of the IPPN Registry. Although studies as the one presented here only become possible by the contribution of multiple pediatric dialysis centers around the globe, regional differences in PD populations and treatment practices might influence observed outcomes. Global adjustment for region, as performed in this study, is generally considered appropriate, although some residual confounding cannot be ruled out. Indeed, we noted a lower prevalence of CAKUT in Asia, more frequent use of diuretics, icodextrin, and biocompatible PD fluids in Europe, and less frequent automated PD use in Turkey. The preliminary conclusion from our multivariate analyses is that residual urine output generally differs between regions, but the region of residence probably does not affect the risk of becoming oligoanuric while on PD.
In summary, in this large pediatric cohort study, we identified that, although substantial urine output is frequently maintained in children undergoing CPD, underlying glomerulopathies and dialysis prescriptions resulting in high ultrafiltration rates are risk factors for rapid progression to oligoanuria. The increased volume and/or duration of urine output associated with the use of diuretics and biocompatible PD fluids are notable observations that might deserve evaluation in controlled interventional trials.
MATERIALS AND METHODS
Data collection
The IPPN Registry collects information from infants, children, and adolescents treated with CPD around the globe. Participation in the registry is voluntary. The participating centers are asked to enroll all prevalent and incident consenting patients and follow them until discontinuation of PD.
Data input to the IPPN Registry is performed exclusively via an Internet-based web platform (www.pedpd.org). Data pertaining to basic patient and PD modality characteristics, growth and weight gain, nutritional modalities, intercurrent hospitalizations, hematology and serum biochemistry, medications, dialysis prescription, daily residual urine volume and ultrafiltration, and, optionally, echocardiography and ambulatory blood pressure monitoring results are submitted every 6 months. In addition, PD-related infections and access revisions and the findings of any Peritoneal Equilibration Tests, and renal and dialytic clearance studies are reported whenever these are performed. Data entries are automatically checked for plausibility and completeness. Data protection is ensured by de-identified data input. The registry protocol was approved by the ethical committees/institutional review boards as required at each participating center. Written parental consent and, whenever appropriate, assent from patients were obtained.
Statistics
Data are expressed by absolute and relative frequencies, mean±s.d. for normally distributed variables, and by median and interquartile range for variables with skewed distribution.
Systolic and diastolic blood pressure was converted to s.d. scores adjusting for age, sex, and height according to the methods published in the NHBPEP Fourth Report.42 Residual GFR was calculated as the mean of urinary creatinine and urea clearance.
Between-group differences were assessed by Pearson's χ2-test, a t-test, or the Wilcoxon rank-sum test as appropriate. Extended Cox proportional hazard modeling was applied, incorporating time-variant covariates and censoring patients with retained diuresis at last observation. For the analysis of diuresis ‘survival' by diuretic use patients was censored when diuretics were discontinued (treatment group) or initiated (no-treatment group). Kaplan–Meier survival analysis with log-rank significance testing was performed to illustrate the effect of key factors associated with preservation of urine output. For the assessment of urine volume over time, general mixed linear models were fitted to account for the dependent data structure within the same patient (random intercept and slope) applying a spatial (spherical) covariance structure based on the Akaike information citerion. Possible covariates were included, and results are given both for full and reduced models. The latter were constructed by backward variable selection with a selection margin of P<0.2. The annualized change in urine volume was calculated from the first and the last observed value and plotted against baseline urine volume within a regression plot including 95% prediction and confidence bands. Data were analyzed using SAS 9.2 (SAS Institute, Cary, NC, USA).
Acknowledgments
We gratefully acknowledge the support by the International Society for Peritoneal Dialysis, Baxter Health Care, Fresenius Medical Care, Ipsen, Pfizer, and IBM. We also appreciate the continued dedicated support of the IPPN by the medical and nursing staff in all collaborating centers.
APPENDIX
The following Principal Investigators are contributors to the IPPN Registry:
Argentina: E. Sojo, Hospital de Pediatria Garrahan, Buenos Aires; P.A. Coccia, Hospital Italiano de Buenos Aires; A. Suarez, Hospital de Nińos Sor. Maria Ludovica La Plata; P.G. Valles, Hospital Pediatrico Humberto Notti, Mendoza; R. Salim, R.S.A. Salta, L. Alconcher, Hospital Interzonal General, Bahia Blanca. Belgium: K. van Hoeck, University Hospital Antwerp, Edegem. Brazil: V. Koch, Instituto da Criança - Hospital das Clinicas FMUSP, Sao Paulo. Canada: J. Feber, Children's Hospital of Eastern Ontario, Ottawa; E. Harvey, Hospital for Sick Children, Toronto; C. White, BC Children's Hospital, Vancouver. Chile: M. Valenzuela, Hospital Guillermo Grant Benavente, Concepcion; J. Villagra, Hospital Base, Osorno; F. Cano, Hospital Luis Calvo Mackenna, Santiago, M.A. Contreras, Roberto del Rio Hospital, Santiago; A. Vogel, Pontivicia Universidad Catolica de Chile, Santiago; P. Zambrano Hospital Dr.Gonzales Cortes, Santiago, P Berrocal Hospital Sotero del Rio, Santiago; P. Hevia, Hospital San Juan de Dios, Santiago. China: M.C. Chiu, Department of Pediatric & Adolescent Medicine, Hong Kong; H. Xu, Children's Hospital of Fudan University, Shanghai. Colombia: J.J. Vanegas, Instituto del Rinon, Medellin; L.M. Higuita, Baxter Servicio al Cliente Colombia, Medellin. Czech Republic: K. Vondrak, University Hospital Motol, Prague. Finland: K. Rönnholm, Hospital for Children and Adolescents, Helsinki; France: B. Ranchin, Hôpital Femme Mčre Enfant, Lyon; G. Roussey, CHU Nantes; T. Ulinski, Armand Trousseau Hospital, Paris; M. Fischbach, Children's Dialysis Center, Strasbourg, J. Harambat, Hopital de Enfants, Bordeaux; Ch. Samaille, Hospital Jeanne de Frandre, Lille; M. Fila, Pediatric Nephrology Unit, Montpellier. Germany: U. Querfeld, Charite Virchow-Klinikum, Berlin; W. Rascher, University Hospital, Erlangen; R. Büscher, Children's Hospital Essen; M. Kemper, University Medical Center, Hamburg; L. Pape, Medical School, Hannover; F. Schaefer, Center for Pediatrics and Adolescent Medicine, Heidelberg; U. John, Kidney Center for Children and Adolescents, Jena; G. Klaus, KfH Kidney Center, Marburg; H. Billing, Children's University Hospital, Tübingen. Greece: F. Papachristou, Aristoteles University, Thessaloniki. Hungary: A. Szabo, Semmelweis University, Budapest. India: A. Bagga, All India Institute of Medical Sciences, New Delhi; M. Kanitkar, Armed Forces Medical College, Pune; R. Sinha, Institute of Child Health, Kolkata; B. Basu, NRS Medical College & Hospital, Kolkata; S. Sethi, The Medicity, Gurgaon. Iran: N. Hooman, Iran University of Medical Sciences, Tehran. Italy: E. Verrina, G. Gaslini Institute, Genova; A. Edefonti, Fondazione Ospedale Maggiore Policlinico, Milano; E. Vidal, Pediatric Nephrology, Dialysis and Transplant Unit, Padova; G. Leozappa, Department of Nefrologia-Urologia, Rome. Israel: D. Landau, Soroka Medical Center, Beer-Sheva. Korea: I.S. Ha, Seoul National University Children's Hospital, Seoul; K.H. Paik, Samsung Medical Center, Seoul. Lebanon: A. Bilal, Rafik Hari University Hospital, Beirut. Macedonia: E. Sahpazova Pediatric Clinic, Skopje. Malaysia: YN Lim, Kuala Lumpur Hospital, Kuala Lumpur. Mexico: L. Sanchez Barbosa, Pediatric Hospital Medial Center SXXI, Cuahutemoc; The Netherlands: J.W. Groothoff, Academic Medical Center, Amsterdam; Y. Konijenberg, Wilhelmina Children's Hospital, Utrecht. New Zealand: W. Wong, Starship Children's Hospital, Auckland. Nicaragua: Y. Silva, Hospital Infantil de Nicaragua, Managua. Oman: M. Al Ryami, Royal Hospital, Muscat. Peru: R. Loza Munarriz, Cayetano Heredia Hospital, Lima. Philippines: Z. Antonio, National Kidney and Transplant Institute, Quezon City. Poland: A.M. Zurowska, D. Borzych, Medical University, Gdansk; D. Drozdz, University Children's Hospital, Krakow; M. Lipka, Children's Memorial Health Institute, Warsaw, Z. Wawer, Public Pediatric Teaching Hospital, Warsaw; M. Sczepanska, Dialysis Division for Children, Zabrze. Romania: O. Brumariu, St. Maria Children's Hospital, Iasi. Saudi Arabia: J. Kari, King Abdul Aziz University Hospital, Jeddah. Singapore: H.K. Yap, Shaw-NKF-NUH Children's Kidney Center. Spain: G. Ariceta, University Hospital Materno-Infantil Vall d'Hebron, Barcelona; M. Aguirre, Hospital de Cruces, Baracaldo, F.Santos, Hospital Universitario Central de Asturias, Oviedo. Turkey: A.S. Bakkaloglu, Hacettepe University, Ankara; S. Bakkaloglu, Gazi University, Ankara; I. Bilge, Department of Pediatric Nephrology, Çapa-Istanbul; L. Sever, Cerrahpasa School of Medicine, Istambul; E. Serdaroglu, Dr.Behcet Children Research and Educational Hospital, Izmir; A. Bal, Tepecik Children and Research Hospital, Izmir; S. Mir, Ege University Faculty of Medicine, Izmir-Bornova. United Arab Emirates: E. Simkova, Dubai Hospital, Dubai. United Kingdom: L. Rees, Great Ormond Street Hospital, London. A.R. Watson, Children & Young People's Kidney Unit, Notthingham. Uruguay: J. Grünberg, SE.N.NI.AD, Montevideo. United States: L. Greenbaum, Children's Healthcare Pediatric Dialysis Unit, Atlanta; A. Neu, Johns Hopkins Hospital, Baltimore; D. Askenazi, Children's Hospital of Alabama, Birmingham; D. Gipson, University of North Carolina, Chapel Hill; H. Patel, Cildren's Hospital, Columbus; A. Al-Akash, Driscoll Children's Hospital, Corpus Christi; S. Pottoore, Children's Medical Center, Dallas; V. Dharnidharka, Division of Pediatric Nephrology, Gainesville; T. Bunchman, Helen DeVos Children's Hospital Grand Rapids; A. Chua, Texas Children's Hospital, Houston; P. Brophy, University of Iowa Children's Hospital, Iowa; B.A.Warady, Children's Mercy Hospital, Kansas City; J. Zaritsky, UCLA Medical Center, Los Angeles; M. Rheault, University of Minnesota Amplatz Children's Hospital, Minneapolis; M. Pradhan, The Children's Hospital of Philadelphia, Philadelphia; N. McAffee, Seattle Children's Hospital, Seattle; L. Burris, Sanford Home Dialysis, Sioux Falls.
All the authors declared no competing interests.
Footnotes
Table S1. Distribution of age, primary renal disease and eGFR at initiation of dialysis in present cohort, all incident patients in IPPN, European children starting PD reported to ESPN/ERA-EDTA Registry, and pediatric patients reported to United States Renal Data System (USRDS).
Table S2. Patient characteristics at commencement of PD according to region of residence.
Supplementary material is linked to the online version of the paper at http://www.nature.com/ki
Supplementary Material
References
- Wang AY, Lai KN. The importance of residual renal function in dialysis patients. Kidney Int. 2006;69:1726–1732. doi: 10.1038/sj.ki.5000382. [DOI] [PubMed] [Google Scholar]
- Marron B, Remon C, Perez-Fontan M, et al. Benefits of preserving residual renal function in peritoneal dialysis. Kidney Int. 2008;73:S42–S51. doi: 10.1038/sj.ki.5002600. [DOI] [PubMed] [Google Scholar]
- Perl J, Bargman JM. The importance of residual kidney function for patients on dialysis: a critical review. Am J Kidney Dis. 2009;53:1068–1081. doi: 10.1053/j.ajkd.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Moist LM, Port FK, Orzol SM, et al. Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol. 2000;11:556–564. doi: 10.1681/ASN.V113556. [DOI] [PubMed] [Google Scholar]
- Hidaka H, Nakao T. Preservation of residual renal function and factors affecting its decline in patients on peritoneal dialysis. Nephrology. 2003;8:184–191. doi: 10.1046/j.1440-1797.2003.00156.x. [DOI] [PubMed] [Google Scholar]
- Caravaca F, Dominguez C, Arrobas M. Predictors of loss of residual renal function in peritoneal dialysis patients. Perit Dial Int. 2002;22:414–417. [PubMed] [Google Scholar]
- Bernardo A, Fonseca I, Rodrigues A, et al. Predictors of residual renal function loss in peritoneal dialysis: is previous renal transplantation a risk factor. Adv Perit Dial. 2009;25:110–114. [PubMed] [Google Scholar]
- Johnson DW, Mudge DW, Sturtevant JM, et al. Predictors of decline of residual renal function in new peritoneal dialysis patients. Perit Dial Int. 2003;23:276–283. [PubMed] [Google Scholar]
- Feber J, Scharer K, Schaefer F, et al. Residual renal function in children on haemodialysis and peritoneal dialysis therapy. Pediatr Nephrol. 1994;8:579–583. doi: 10.1007/BF00858132. [DOI] [PubMed] [Google Scholar]
- Michels WM, Verduijn M, Grootendorst DC, et al. Decline in residual renal function in automated compared with continuous ambulatory peritoneal dialysis. Clin J Am Soc Nephrol. 2011;6:537–542. doi: 10.2215/CJN.00470110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufnagel G, Michel C, Queffeulou G, et al. The influence of automated peritoneal dialysis on the decrease in residual renal function. Nephrol Dial Transplant. 1999;14:1224–1228. doi: 10.1093/ndt/14.5.1224. [DOI] [PubMed] [Google Scholar]
- Hiroshige K, Yuu K, Soejima M, et al. Rapid decline of residual renal function in patients on automated peritoneal dialysis. Perit Dial Int. 1996;16:307–315. [PubMed] [Google Scholar]
- Singhal MK, Bhaskaran S, Vidgen E, et al. Rate of decline of residual renal function in patients on continuous peritoneal dialysis and factors affecting it. Perit Dial Int. 2000;20:429–438. [PubMed] [Google Scholar]
- Liao CT, Chen YM, Shiao CC, et al. Rate of decline of residual renal function is associated with all-cause mortality and technique failure in patients on long-term peritoneal dialysis. Nephrol Dial Transplant. 2009;24:2909–2914. doi: 10.1093/ndt/gfp056. [DOI] [PubMed] [Google Scholar]
- Drechsler C, de Mutsert R, Grootendorst DC, et al. Association of body mass index with decline in residual kidney function after initiation of dialysis. Am J Kidney Dis. 2009;53:1014–1023. doi: 10.1053/j.ajkd.2008.11.027. [DOI] [PubMed] [Google Scholar]
- Bragg-Gresham JL, Fissell RB, Mason NA, et al. Diuretic use, residual renal function, and mortality among hemodialysis patients in the Dialysis Outcomes and Practice Pattern Study (DOPPS) Am J Kidney Dis. 2007;49:426–431. doi: 10.1053/j.ajkd.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Herget-Rosenthal S, von Ostrowski M, Kribben A. Definition and risk factors of rapidly declining residual renal function in peritoneal dialysis: an observational study. Kidney Blood Press Res. 2012;35:233–241. doi: 10.1159/000332887. [DOI] [PubMed] [Google Scholar]
- Shin SK, Noh H, Kang SW, et al. Risk factors influencing the decline of residual renal function in continuous ambulatory peritoneal dialysis patients. Perit Dial Int. 1999;19:138–142. [PubMed] [Google Scholar]
- Coronel F, Perez-Flores I, Calvo N, et al. Impact of cardiovascular events on residual renal function during the first year of peritoneal dialysis. Perit Dial. 2007;27:454–456. [PubMed] [Google Scholar]
- Cho Y, Johnson DW, Badve SV, et al. The impact of neutral-pH peritoneal dialysates with reduced glucose degradation products on clinical outcomes in peritoneal dialysis patients. Kidney Int. 2013;84:969–979. doi: 10.1038/ki.2013.190. [DOI] [PubMed] [Google Scholar]
- Shemin D, Bostom AG, Lambert C, et al. Residual renal function in a large cohort of peritoneal dialysis patients: change over time, impact on mortality and nutrition. Perit Dial Int. 2000;20:439–444. [PubMed] [Google Scholar]
- Chadha V, Blowey DL, Warady BA. Is growth a valid outcome measure of dialysis clearance in children undergoing peritoneal dialysis. Perit Dial Int. 2001;21:S179–S184. [PubMed] [Google Scholar]
- Bakkaloglu SA, Saygili A, Sever L, et al. Assessment of cardiovascular risk in paediatric peritoneal dialysis patients: a Turkish Pediatric Peritoneal Dialysis Study Group (TUPEPD) report. Nephrol Dial Transplant. 2009;24:3525–3532. doi: 10.1093/ndt/gfp297. [DOI] [PubMed] [Google Scholar]
- Guzzo I, Mancini E, Wafo SK, et al. Residual renal function and nutrition in young patients on chronic hemodialysis. Pediatr Nephrol. 2009;24:1391–1397. doi: 10.1007/s00467-009-1144-7. [DOI] [PubMed] [Google Scholar]
- Lang SM, Bergner A, Topfer M, et al. Preservation of residual renal function in dialysis patients: effects of dialysis-technique-related factors. Perit Dial Int. 2001;21:52–57. [PubMed] [Google Scholar]
- Misra M, Vonesh E, Van Stone JC, et al. Effect of cause and time of dropout on the residual GFR: a comparative analysis of the decline of GFR on dialysis. Kidney Int. 2001;59:754–763. doi: 10.1046/j.1523-1755.2001.059002754.x. [DOI] [PubMed] [Google Scholar]
- Lysaght MJ, Vonesh EF, Gotch F, et al. The influence of dialysis treatment modality on the decline of remaining renal function. ASAIO Trans. 1991;37:598–604. [PubMed] [Google Scholar]
- Borzych-Duzalka D, Bilginer Y, Ha IS, et al. Management of anemia in children receiving chronic peritoneal dialysis. J Am Soc Nephrol. 2013;24:665–676. doi: 10.1681/ASN.2012050433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medcalf JF, Harris KP, Walls J. Role of diuretics in the preservation of residual renal function in patients on continuous ambulatory peritoneal dialysis. Kidney Int. 2001;59:1128–1133. doi: 10.1046/j.1523-1755.2001.0590031128.x. [DOI] [PubMed] [Google Scholar]
- Liao CT, Shiao CC, Huang JW, et al. Predictors of faster decline of residual renal function in Taiwanese peritoneal dialysis patients. Perit Dial Int. 2008;28 (Suppl 3:S191–S195. [PubMed] [Google Scholar]
- van Olden RW, van Meyel JJ, Gerlag PG. Acute and long-term effects of therapy with high-dose furosemide in chronic hemodialysis patients. Am J Nephrol. 1992;12:351–356. doi: 10.1159/000168471. [DOI] [PubMed] [Google Scholar]
- Takatori Y, Akagi S, Sugiyama H, et al. Icodextrin increases technique survival rate in peritoneal dialysis patients with diabetic nephropathy by improving body fluid management: a randomized controlled trial. Clin J Am Soc Nephrol. 2011;6:1337–1344. doi: 10.2215/CJN.10041110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y, Johnson DW, Craig JC, et al. Biocompatible dialysis fluids for peritoneal dialysis. Cochrane Database Syst Rev. 2014;3:CD007554. doi: 10.1002/14651858.CD007554.pub2. [DOI] [PubMed] [Google Scholar]
- Johnson DW, Brown FG, Clarke M, et al. Effects of biocompatible versus standard fluid on peritoneal dialysis outcomes. J Am Soc Nephrol. 2012;23:1097–1107. doi: 10.1681/ASN.2011121201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui SL, Yung S, Yim A, et al. A combination of biocompatible peritoneal dialysis solutions and residual renal function, peritoneal transport, and inflammation markers: a randomized clinical trial. Am J Kidney Dis. 2012;60:966–975. doi: 10.1053/j.ajkd.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Fan SL, Pile T, Punzalan S, et al. Randomized controlled study of biocompatible peritoneal dialysis solutions: effect on residual renal function. Kidney Int. 2008;73:200–206. doi: 10.1038/sj.ki.5002574. [DOI] [PubMed] [Google Scholar]
- Kim S, Oh J, Kim S, et al. Benefits of biocompatible PD fluid for preservation of residual renal function in incident CAPD patients: a 1-year study. Nephrol Dial Transplant. 2009;24:2899–2908. doi: 10.1093/ndt/gfp054. [DOI] [PubMed] [Google Scholar]
- Haag-Weber M, Kramer R, Haake R, et al. Low-GDP fluid (Gambrosol trio) attenuates decline of residual renal function in PD patients: a prospective randomized study. Nephrol Dial Transplant. 2010;25:2288–2296. doi: 10.1093/ndt/gfq087. [DOI] [PubMed] [Google Scholar]
- Wuhl E, Trivelli A, Picca S, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639–1650. doi: 10.1056/NEJMoa0902066. [DOI] [PubMed] [Google Scholar]
- Jafar TH, Stark PC, Schmid CH, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med. 2003;139:244–252. doi: 10.7326/0003-4819-139-4-200308190-00006. [DOI] [PubMed] [Google Scholar]
- Harambat J, van Stralen KJ, Schaefer F, et al. Disparities in policies, practices and rates of pediatric kidney transplantation in Europe. Am J Transplant. 2013;13:2066–2074. doi: 10.1111/ajt.12288. [DOI] [PubMed] [Google Scholar]
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.