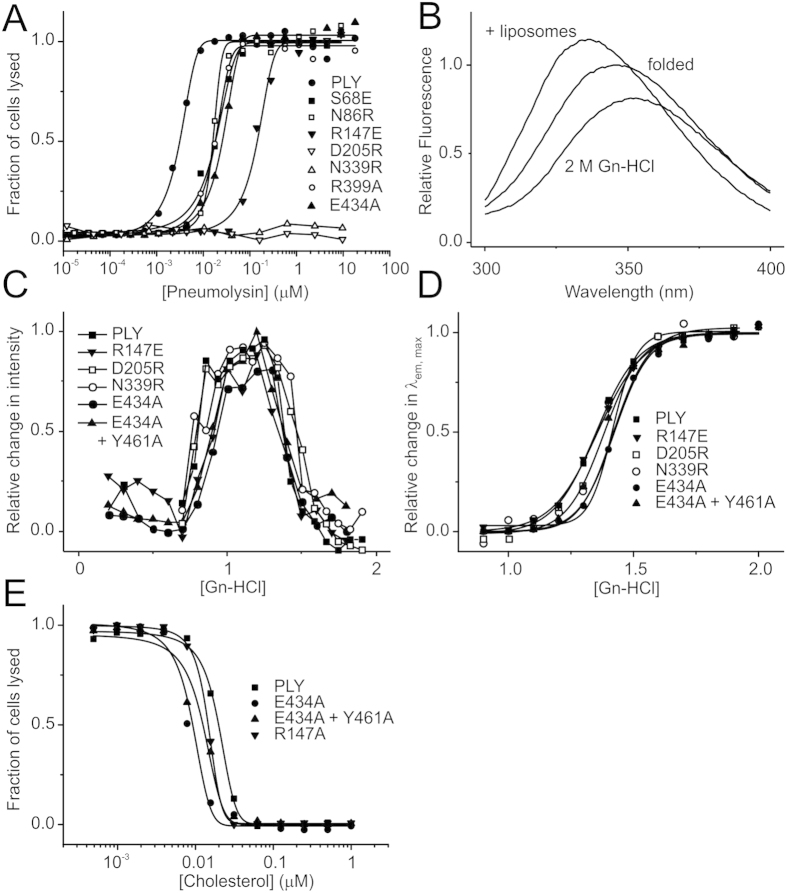
Figure 4. Hemolytic activities and biophysical properties of mutant pneumolysins.
(A) hemolysis of sheep erythrocytes by selected pneumolysin mutants. (B) Fluorescence spectra of folded and unfolded pneumolysin (in 2M guanidine-HCl) and pneumolysin with liposomes. The fluorescence spectra of all mutants were comparable to that of the wild-type and exhibited similar changes with respect to the fluorescence intensity and λem, max. (C,D), denaturation of wild-type and mutant pneumolysins measured by changes in fluorescence intensity (C) and λem, max (D). The unfolding transition measured by the change in λem, max corresponded approximately to the decrease in fluorescence observed in (C). (E) cholesterol-mediated inhibition of haemolysis by wild-type and mutant pneumolysins. Data are average of six measurements.