Figure 9.
Nucleolytic Activity of TSN Is Required for the Proper Dynamics of Rbp47b in SGs.
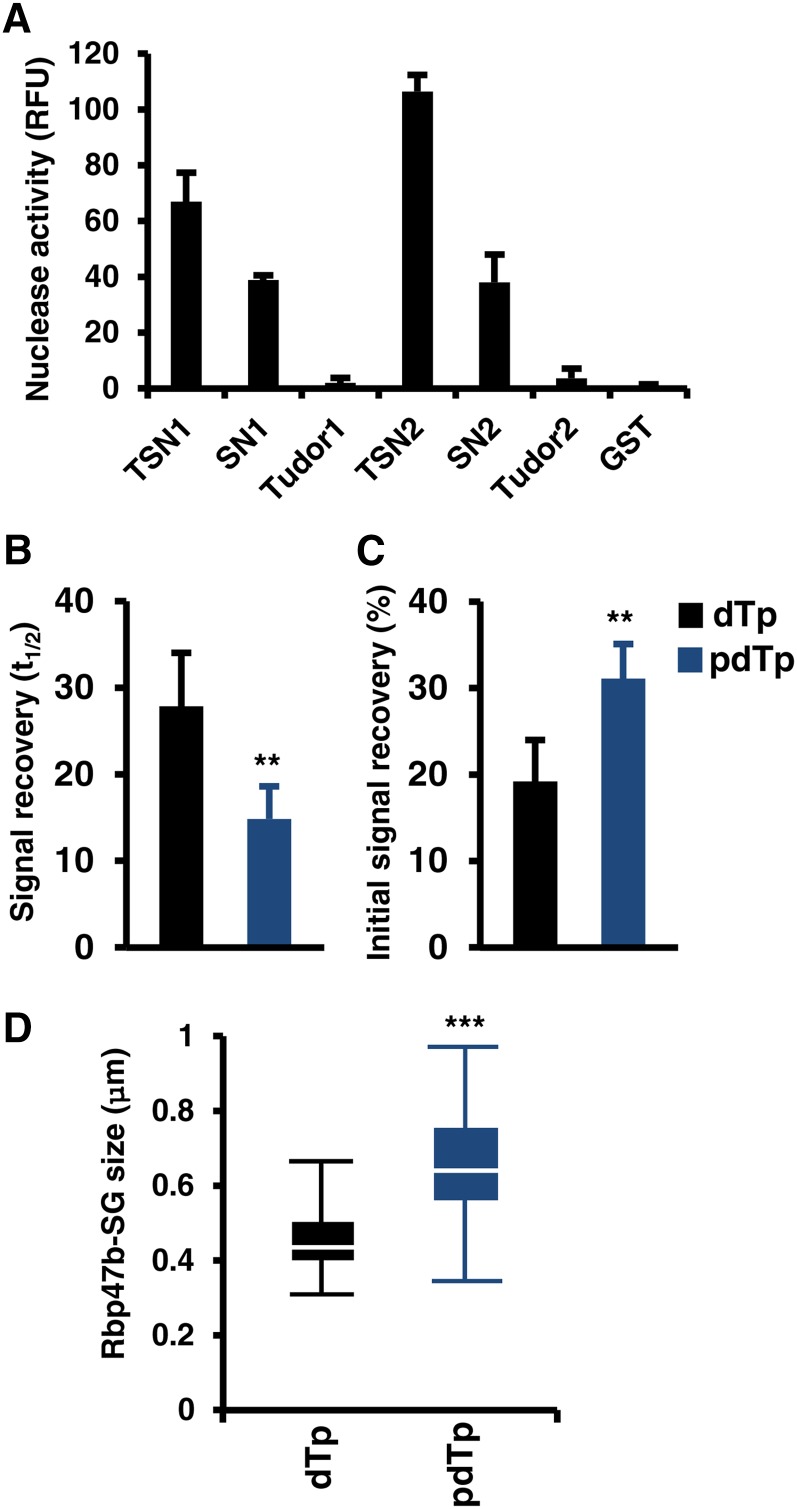
(A) In vitro nucleolytic activity of full-length and truncated forms of TSN1 and TSN2. Recombinant GST fusion proteins were purified from E. coli and incubated in nuclease buffer containing fluorescent RNA substrate. RNase activity was determined by a fluorometric assay. GST was used as a negative control. RFU, relative fluorescence units. Data show means ± sd of three independent experiments.
(B) and (C) Signal recovery (t1/2; [B]) and initial signal recovery (%; [C]) of RFP-Rbp47b determined by FRAP analysis in Arabidopsis root tip cells treated with pdTp and dTp. Five-day-old seedlings expressing Pro35S:GFP-Rbp47b were incubated in MS medium supplemented with 50 μM pdTp or dTp (control) for 5 h at 23°C and then heat-stressed for 40 min at 39°C. **P < 0.01, Student’s t test. Data show means ± sd of triplicate experiments, each containing at least 10 seedlings.
(D) Box plot of the size of GFP-Rbp47b foci (SGs) in root tip cells of plants treated with pdTp and dTp and then subjected to heat stress for 40 min at 39°C. Each box plot shows the median (solid line), the 25th and 75th percentiles (boxes), and the 5th and 95th percentiles (error bars). ***P < 0.001, Student’s t test.