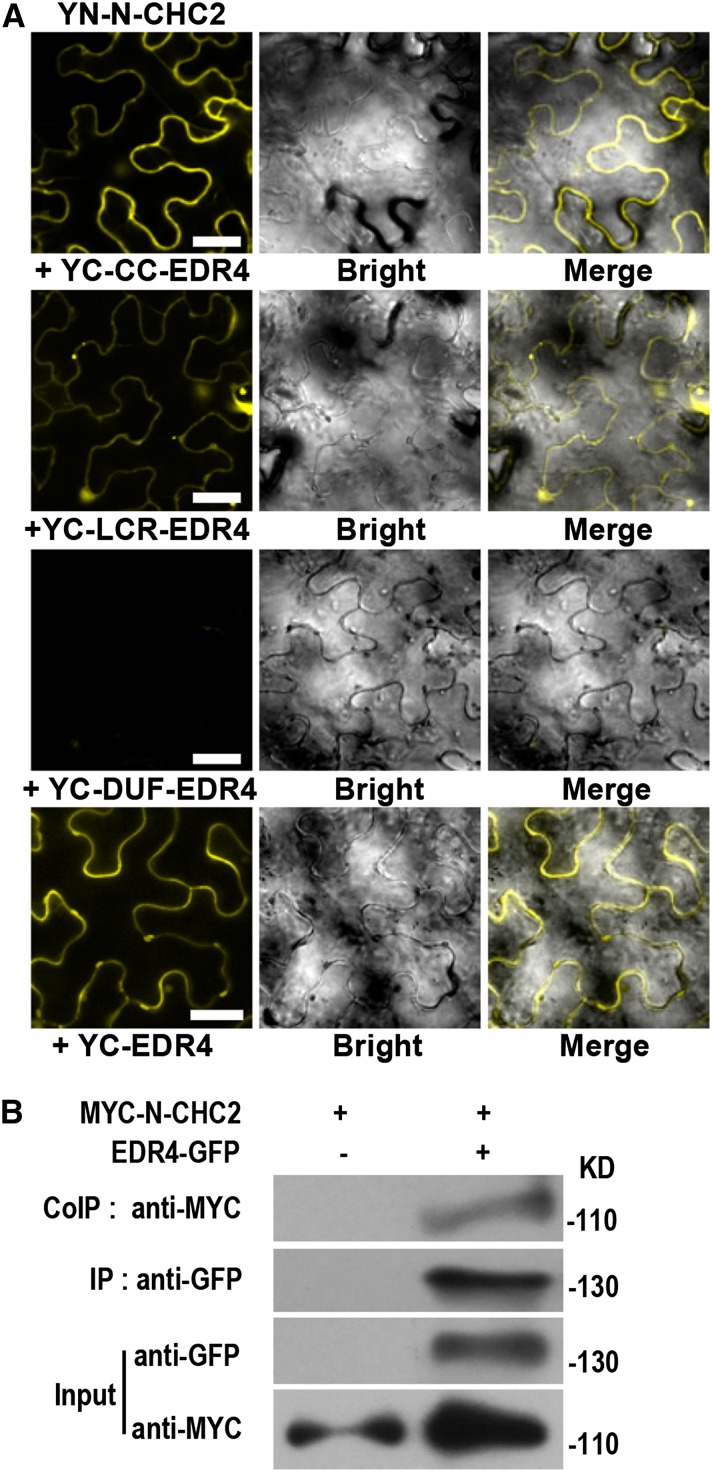
Figure 8.
EDR4 Interacts with CHC2 in Vivo.
(A) BiFC assays in N. benthamiana show interactions between YN-N-CHC2 and YC-EDR4. The N-terminal domain (amino acids 1 to 1016) of CHC2 was fused to the N-terminal fragment of YFP, and the fragments, including full-length EDR4, the CC domain, four LCR domains, and the Duf3133 domain of EDR4, were fused to the C-terminal fragment of YFP. YFP fluorescence indicates an interaction between the two proteins. The experiments were repeated three times with similar results. Bars = 30 μm.
(B) Expression and Co-IP of pEDR4-EDR4-GFP and 35S-MYC-N-CHC2 in Arabidopsis. Total protein was extracted from 4-week-old transgenic plants expressing both EDR4-GFP and MYC-N-CHC2. The EDR4-GFP protein was immunoprecipitated with anti-GFP antibody, and the presence of MYC-N-CHC2 protein was detected by immunoblot analysis with anti-MYC antibody. The experiments were repeated three times with similar results.