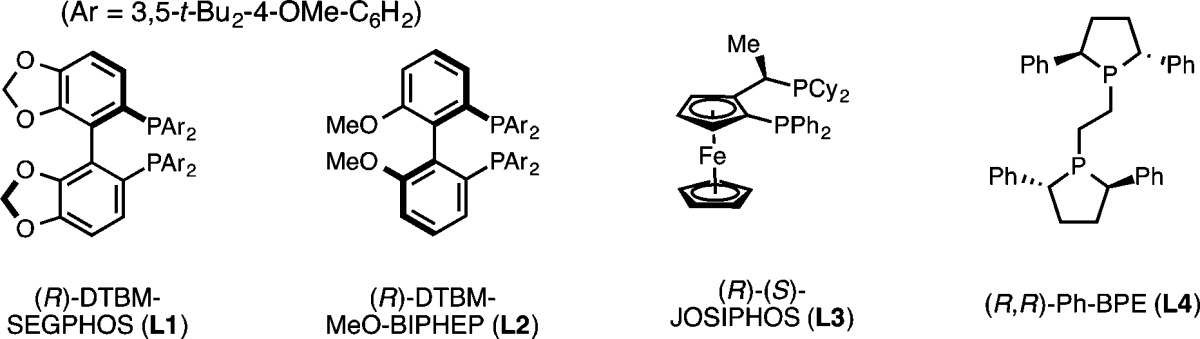
Table 1. Optimization of Reaction Conditions.
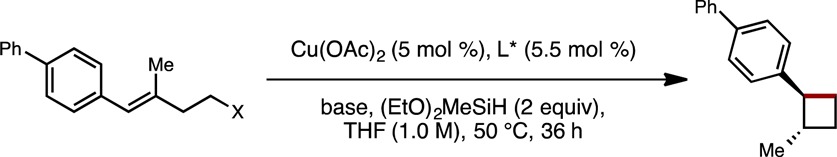
| entry | X | L* | base (equiv) | % yielda (% eeb) |
|---|---|---|---|---|
| Effect of Base | ||||
| 1 | OMs | L1 | KOt-Bu (2.0) | 0 |
| 2 | OMs | L1 | LiOt-Bu (2.0) | 0 |
| 3 | OMs | L1 | LiOMe (2.0) | 17 (96) |
| 4 | OMs | L1 | NaOMe (2.0) | 0 |
| Effect of Leaving Group | ||||
| 5 | OTs | L1 | LiOMe (2.0) | 9 |
| 6 | Br | L1 | LiOMe (2.0) | 16 |
| 7 | Br | L1 | LiOMe (4.0) | 70 (97) |
| 8 | OMs | L1 | LiOMe (4.0) | 14 |
| Effect of Ligand | ||||
| 9 | Br | L2 | LiOMe (4.0) | 16 |
| 10 | Br | L3 | LiOMe (4.0) | 8 |
| 11 | Br | L4 | LiOMe (4.0) | 5 |
| 12 | Br | L1 | LiOMe (4.0)c | 92d (99) |
NMR yield with 1,3,5-trimethoxybenzene as internal standard, 0.1 mmol scale.
ee determined by chiral HPLC.
4.0 equiv (MeO)2MeSiH at 2.0 M in THF, 55 °C.
83% isolated yield, 0.5 mmol scale.