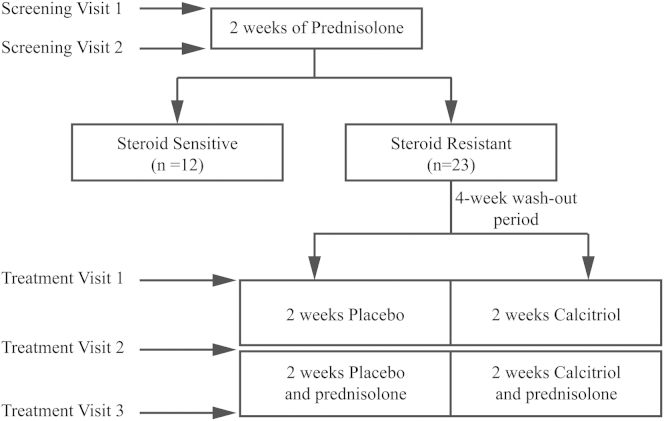
Fig E1.
Clinical trial schematic. Patients with severe asthma were recruited and given prednisolone at screening visit 1 and returned 2 weeks later at screening visit 2 when patients with SS asthma were excluded from the trial and patients with SR asthma were retained. After a 4-week washout period, the patients with SR asthma were randomly assigned to receive calcitriol or placebo for 1 month (treatment visit 1). After 2 weeks (treatment visit 2), the patients were started on additional oral prednisolone and then returned 2 weeks later for the final visit (treatment visit 3). Lung function was assessed at all clinical trial visits except treatment visit 1. CD8-depleted PBMCs were assessed from peripheral blood collected at screening visit 1 and treatment visit 3.