Figure 4.
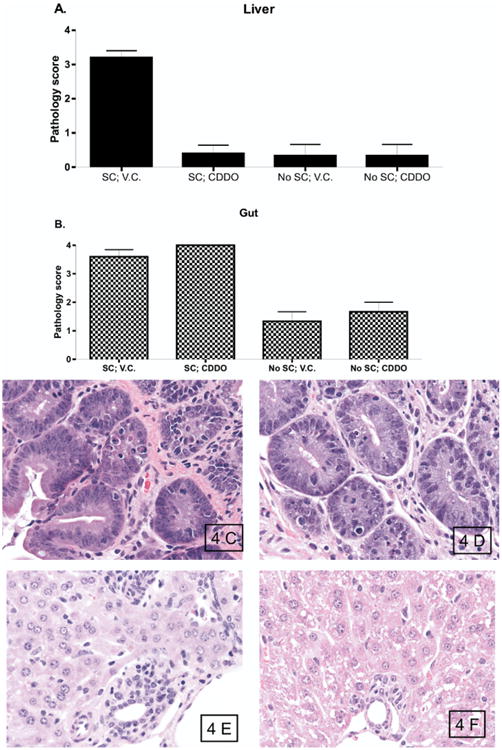
CDDO administration results in decreased hepatic injury but not intestinal injury in mice with GVHD. B6 (H2b) mice received TBI (950 cGy), followed by infusion of 10 × 106 BALB/c (H2d)BMC and 35 × 106 BALB/c SC. Mice were treated with vehicle control or CDDO (240 μg/dose) three times a day intraperitoneally on day 0 and day +1 for a total of 2 doses. (A, B). Pathology scores in the liver (A) and gut (B) of mice that received BMC alone or with splenocytes, followed by administration of vehicle control or CDDO. (C, D) Photomicrographs of representative gut tissue sections. Mice that received BMC with spleen cells followed by vehicle control (C) and mice that received BMC with spleen cells followed by CDDO (D) demonstrated similar prominent tissue damage with numerous apoptotic bodies. (E, F). Photomicrographs of representative liver tissue sections. Mice that received BMC with spleen cells followed by vehicle control (E) demonstrated expensive GVHD with bile duct proliferation, vasculitis, lobular hepatitis, and numerous apoptotic cells. In contrast, mice that received BMC with spleen cells followed by CDDO (F) demonstrated preserved portal triad architecture, no vasculitis, no lobular hepatitis, and virtually no apoptotic cells. H&E staining, original magnification ×500.
