Figure 5. RNP dimers are the basic stabilization units of the IBDV capsid.
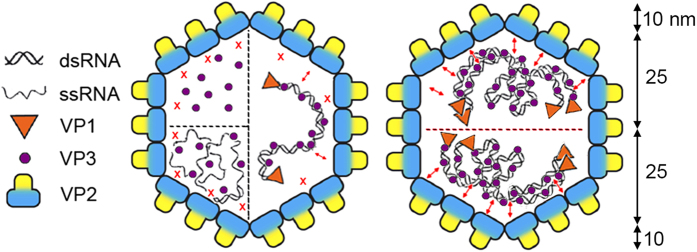
Scheme of the IBDV capsid showing structural components involved in stabilizing interactions. When VP3 is found alone inside the capsid (as in empty VLP, E1 and E2) or in the presence of ssRNA (as in full VLP), contacts with VP2 (red X) are weak or non-existent. When dsRNA is present, these contacts are more intense (small double arrowheads), presumably because dsRNA-bound VP3 acquire the correct conformation to establish a stronger interaction with VP2 (left). Mature IBDV virions, represented by E5-E6 capsids, are polyploid and contain four packaged dsRNA segments organized as RNP. In this case, RNP are organized internally as dimers; in this oligomeric state, VP3 acquires a conformation with which it establishes strong interactions with the inner surface of the viral shell. This structural organization of the genome explains the stepwise disruption profile during mechanical fatigue (shell thickness is ~10 nm based on three-dimensional cryo-EM analysis).
