Abstract
Introduction
Obesity is thought to exert detrimental effects on the cardiovascular (CV) system. However, this relationship is impacted by the co-occurrence of CV risk factors, type II diabetes (T2DM), and overt disease. We examined the relationships between obesity, assessed by body mass index (BMI) and waist circumference (WC), and CV function in 102 subjects without overt CV disease. We hypothesized that obesity would be independently predictive of CV remodeling and functional differences, especially at peak exercise.
Methods
Brachial (bSBP) and central (cSBP) systolic pressure, carotid-to-femoral pulse wave velocity (PWVcf) augmentation index (AGI) (by SphygmoCor), and carotid remodeling (B-mode ultrasound) were examined at rest. Further, peak exercise cardiac imaging (Doppler ultrasound) was performed to measure the coupling between the heart and arterial system.
Results
In backward elimination regression models, accounting for CV risk factors, neither BMI nor WC were predictors of carotid thickness or PWVcf; rather age, triglycerides, and hypertension were the main determinants. However, BMI and WC predicted carotid cross-sectional area and lumen diameter. When examining the relationship between body size and SBP, BMI (β=0.32) and WC (β=0.25) were predictors of bSBP (p<0.05), whereas, BMI was the only predictor of cSBP (β=0.22, p<0.05) indicating a differential relationship between cSBP, bSBP and body size. Further, BMI (β=−0.26) and WC (β=−0.27) were independent predictors of AGI (p<0.05). As for resting cardiac diastolic function, WC seemed to be a better predictor than BMI. However, both BMI and WC were inversely and independently related to arterial elastance (net arterial load) and end-systolic elastance (cardiac contractility) at rest and peak exercise.
Discussion
These findings illustrate that obesity, without T2DM and overt CV disease, and after accounting for CV risk factors, is susceptible to pathophysiological adaptations that may predispose individuals to an increased risk of CV events.
Keywords: Obesity, Cardiovascular Dysfunction, Exercise
Introduction
Obesity represents a disease of epidemic proportions with 36% of adults in United States presenting with obesity1. The health consequences with obesity are numerous with an increased risk of cardiovascular (CV) events2. Impairments in CV function have been reported with obesity3-8. While these studies provide important information on the pathophysiological associations with obesity, the obese populations examined tended to present with type 2 diabetes mellitus (T2DM) and overt CV disease. Further, often the co-occurrence of CV risk factors was not taken into account when examining the CV associations with obesity. Thus, the reported associations may reflect the contribution of comorbidities to the CV dysfunction. Identification of the early manifestations of CV dysfunction prior to the development of T2DM and overt CV disease may represent a critical window for interventions aimed at preventing the development of chronic CV adaptations that expose obese subjects to various CV pathologies, including heart failure9.
The interplay between the arterial system and the left ventricle (LV), known as arterial-ventricular coupling, has an important role in CV performance10, and is a predictor of CV mortality11. Indeed, a mismatch in arterial-ventricular coupling has been implicated as a pathway leading to heart failure12. It is therefore important to understand the interplay between the arterial system and LV to fully comprehend the impact of obesity on CV function and its associated risk with CV events. To date, most of the obesity-related CV associations have been limited to resting measures. Examining CV associations during exercise would provide further pathophysiological insights into the effects of obesity.
The aim of this study was to examine the relationship between obesity and measurements of LV and arterial structure/function at rest and peak exercise, independent of CV risk factors, in a population free of T2DM and overt CV disease. We hypothesized that obesity will be independently predictive of CV remodeling and functional differences, especially at peak exercise. However, common methods for the quantification of obesity, namely body mass index (BMI) and waist circumference (WC) reflect different aspects of obesity. BMI is regarded as a general measure of obesity that has poor sensitivity for calculating percentage body fat (%BF)13. Whereas, WC is highly correlated with intra-abdominal fat volumes14 and predicts increased mortality beyond that accounted for by BMI15. As such, BMI may under represent the epidemic16, 17 and the physiological associations to the CV system. Therefore, the second aim of the study was to compare relationship between the various measures of obesity (I,e, BMI, WC and %BF) and cardiovascular function. We expected that the WC would be better suited for the assessment of CV dysfunction compared to BMI and %BF.
Methods
Study Subjects
Subjects were recruited from the Morgantown, WV area in response to advertisements for CV assessments. The total study sample comprised of 102 subjects (33 men and 69 women) between 22-70 years, who were free from T2DM and overt CV disease as determined by a detailed medical history, physical examination, and a normal resting electrocardiogram. Further, none of the subjects were current or former smokers. Exclusion criteria included pulmonary disease, angina, atrial fibrillation, aortic stenosis, anemia, myocardial infarction, stroke, or coronary revascularization as assessed by a detailed medical history, and physical examination including the examination of a carotid ultrasound. Subjects who participated in regular exercise (>30 mins/day, 3 times/week) were excluded to ensure similar physical activity levels between subjects. All subjects provided written informed consent to participate that was approved by WVU Institutional Review Board.
Study Design
CV assessments were performed between 7:00-10:00 AM, in a quiet, temperature-controlled room, after a 12-hr fast and abstinence from alcohol, caffeine, and vitamins. CV medications were withheld 24 hrs prior to assessments. After 15 mins of quiet rest subjects underwent resting supine non-invasive assessments of CV structure and function. Once supine assessments were completed, subjects performed a maximal exercise stress test to measure peak CV function.
Body anthropometry
Height and weight, from which BMI (weight/height2), were measured using standard laboratory procedures. Fat distribution was assessed by measuring the WC at the site of the smallest circumference between the rib cage and the ileac crest, with the subjects in standing position. Percentage of body fat (%BF) was calculated from body volume by the BodPod® (Life measurement, Concord, CA, USA). Subjects wore tightly fitting bathing suits and a swim cap during the volume-measurements in the BodPod®. Of note, %BF was available on 84 of the 102 subjects.
Metabolic indices
Blood sampling was performed in the morning after a 12-hr fast. Blood was drawn locally and processed at WVU Hospital’s central laboratory in Morgantown, WV. High-density lipoprotein (HDL) cholesterol, triglycerides (Tg), glucose, and insulin were determined using standard clinical laboratory techniques. Homeostasis model assessment of insulin resistance (HOMA-IR) was estimated with the following formula: insulin resistance = fasting plasma insulin (μU/mL) × fasting plasma glucose (mmol/L) / 22.5.
Cardiovascular measurements
Blood pressure and pulse wave analysis
Automated (Critikon Dinamap Compact BP monitor, GE Medical, FL, USA) brachial systolic (bSBP) and diastolic (bDBP) BP were measured from which pulse pressure (bPP) was calculated (bSBP-bDBP). As previously described in detail18, pulse wave analysis was performed noninvasively on the radial artery (SphygmoCor system, AtCor Medical, Sydney, Australia). Central BP (i.e., cSBP and cPP) were estimated using a generalized transfer function and customized software (SphygmoCor system). Central augmented pressure (AP) was defined as the difference between cSBP and the pressure at the forward wave peak. Central augmentation index (AGI) was calculated AP/cPP*100 and adjusted to a ‘standard HR’ of 75 beats/min (AGI@75).
Arterial geometry and stiffness
In the supine position, ultrasound (GE Vivid i) 2-D images of the right common carotid artery (CCA) were obtained 1-2 cm proximal to the carotid bifurcation to measure maximal lumen diameter (CDd), and intima-medial thickness (cIMT). CCA cross-sectional area was calculated as cCSA = [(CDd/2)2xπ]-(CDd/2-cIMT)2xπ). Carotid-to-femoral pulse wave velocity (PWVcf; central arterial stiffness) was measured by applanation tonometry (AtCor Medical, Sydney, Australia). ECG-gated waveforms were sequentially recorded. Aortic distance was calculated as the difference in the distances from the carotid to the suprasternal notch and from the suprasternal notch to the femoral artery. Time delay was calculated using a foot-of-the-wave method.
Exercise Capacity
Subjects exercised to exhaustion on a modified monarch bike, which allowed the subject to exercise in an upright position while assisting the sonographer with the acquisition of optimal echo images. During exercise the echo images were acquired approximately 60-90 seconds into each 3-min stage. If all images were not acquired within the time frame the duration of the exercise stage was extended to acquire those images. Pedal speed was set at 50 rpm, and workloads increased by 25 W every 3 min until exhaustion. Peak oxygen consumed (VO2peak) was measured from breath-by-breath gas analysis (ParvoMedics).
Echocardiography
Echocardiograms were performed by registered diagnostic cardiac sonographers (GE Vivid I, GE Healthcare, UK). As previously described from our laboratory19, resting standard 2-D images, continuous/pulse wave and spectral tissue Doppler tracings were obtained. During exercise, 2-D image of the parasternal long axis view were obtained for LV outflow tract diameter, quickly followed by 4-chamber (for cardiac volumes and mitral flow velocities) and 5-chamber views (for pulse-and continuous-wave Doppler-flow tracings). CV measurements were performed offline, by a single investigator and the intra-class correlation coefficient (>0.80) have been previously reported19.
Diastolic function
In the supine position, diastolic function was assessed by pulse wave Doppler examination of mitral inflow early (E) and late (A) velocities, and their ratio E/A. Tissue Doppler systolic (s’), early (e’), and late (a’) diastolic velocities of the lateral mitral annulus were recorded, from which the é/á and E/e’ ratios were calculated. End-diastolic pressure was estimated as 11.96+0.596xE/e’. Pulse wave Doppler examination of pulmonary venous flow was used to measure peak systolic (S) velocity, peak anterograde diastolic (D) velocity, and their ratio S/D, which is an estimate of left atrial reservoir function20.
Arterial-ventricular coupling
Resting and peak exercise arterial-ventricular coupling was calculated as: 1) arterial elastance index (EaI = end-systolic pressure/stroke volume) a measure of the net arterial load; 2) LV end-systolic elastance index (EesI), a measure of LV contractility and chamber performance, was calculated from BP, stroke volume, ejection fraction, and pre-ejection and systolic ejection time intervals from LV outflow Doppler, determined by the validated single-beat technique10; 3) arterial-ventricular coupling ratio = EaI/EesI. A detailed description of these equations have previously been reported from our laboratory19.
Scaling for Body Size
Scaling of physiological measures for body size is essential when examining the effects of obesity on physiological parameters. Hemodynamic indices that depend on volume are influenced by body size, and therefore should be normalized to body surface area (BSA). Appropriate scaling of CV parameters to body size may represent either ratiometric or allometric scaling21, 22. According to previous analyses that examined the normal physiological (only healthy subjects without any CVD and with a BMI between 18-25 kg/m2) relationship between body size and Ea/Ees and found that Ea, and Ees were linearly related to BSA21. Thus, Ea and Ees were indexed (noted by I, i.e., EesI and EaI) ratiometrically to BSA, whereas their ratio Ea/Ees is a dimensionless parameter. Further, the ratiometric adjustment of VO2peak to body weight was found to be acceptable in adults23.
Statistical analysis
Measurements of CV function were performed offline by a single investigator who was blinded to group allocation. The intra-class correlation coefficient (ICC) and coefficient of variations for all echocardiographic variables were derived in a subset of subjects (n=8-17). At rest, the ICC and the coefficient of variation for all variables, collected on two separate days, was >0.80 or between 7-12%, respectively. In particular, cfPWV (coefficient of variation =3% and ICC = 0.98), cIMT (coefficient of variation = 3% and ICC = 0.96), AP (coefficient of variation = 6% and ICC = 0.99), and AGI (coefficient of variation = 6% and ICC = 0.97). Similar results were obtained for echocardographic variables evaluated during peak exercise with all variables having an ICC>0.80 and coefficient of variation between 7-12% with the exception of the arterial-ventricular coupling ratio (ICC=0.63).
We examined the relationship between obesity and CV function in two forms: 1) by assigning individuals into BMI and WC groups; and 2) through examination of BMI and WC as continuous functions in multi-linear regression models. BMI groups were defined as normal 18-24.99 kg/m2; overweight 25.0-29.9 kg/m2; and obese ≥30 kg/m2 24. WC groups were defined as non-risk (group 1: Male<102, Female<88) and at-risk (group 2: Male≥102, Female≥88). Differences between BMI groups were analyzed by a one-way ANOVA, or Krustal Wallis Test with a post-hoc test, whereas comparisons between WC groups were analyzed by an independent t-test or Mann-Whitney test. The changes from rest to peak exercise in EaI, EesI, and EaI/EesI were examined by a time (rest to peak exercise) by group (BMI or WC) interaction evaluated using a two-way repeated-measures ANOVA with Tukey’s post-hoc test. Stepwise backwards linear regression models (removal criteria was set at a p<0.1) were used to determine whether BMI ,WC, or %BF was a predictor of the CV parameters after adjusting for confounding risk factors, such as age, sex, fasting glucose, insulin (or HOMA-IR), Tg (or HDL, or the Tg/HDL ratio), and hypertension (HTN) status. HTN was defined as a SBP >140 mmHg or DBP >90 mmHg, or subject’s self-reported history of HTN, or anti-HTN medications. We also added to the final backward elimination models a BMI (WC or %BF) by sex interaction term to establish if sex differences existed in the relationship between obesity and CV parameters. As, BMI, WC and %BF were highly correlated these variables were examined separately in the regression models. Data are presented as means ± SEM and p≤0.05 was required for significance. All analyses were performed using SPSS version 20 (SPSS Inc, Chicago, Illinois).
Results
As evident in Table 1, obese subjects were characterized by a higher BMI or WC, higher Tg, glucose (for BMI only), insulin, Tg/HDL, and lower HDL concentrations. Hypertension was present in 30% of subjects, with 84% of those subjects on HTN medications. Furthermore, obese subjects had a greater prevalence of HTN (54% vs. 3%) and HTN medications (43% vs. 3%) vs. normal weight individuals (Table 1). However, similar ejection fractions were evident between obesity groups.
Table 1.
Subject characteristics
| BMI Group | WC Group | ||||
|---|---|---|---|---|---|
| Normal | Overweight | Obese | Non-risk | At-risk | |
|
|
|||||
| Number of subjects | 34 | 26 | 42 | 52 | 50 |
| Sex, % Female | 71 | 62 | 69 | 60 | 76* |
| Age, years | 43±2 | 46±3 | 49±2 | 43±2 | 50±2 |
| Weight, kg | 64±1 | 76±2* | 102±2*# | 68±2 | 98±3* |
| Height, cm | 169±1 | 166±2 | 167±1 | 167±1 | 168±1 |
| BMI, kg/m2 | 22±0.3 | 28±0.3* | 37±0.8*# | 24±0.5 | 35±0.9* |
| Waist, cm | 79±1 | 90±2* | 112±2*# | 82±1 | 109±2* |
| Triglycerides, mg/dL | 65±5 | 125±15 | 158±22* | 88±9 | 150±19* |
| HDL, mg/dL | 62±3 | 49±3* | 48±3* | 57±3 | 49±2* |
| Glucose, mg/dL | 92±2 | 97±2 | 100±1* | 95±1 | 98±2 |
| Insulin, μIU/mL | 4±0.4 | 10±1.3* | 13±1.0* | 6±0.7 | 12±1* |
| HOMA-IR | 1.01±0.12 | 2.42±0.37* | 3.19±0.29* | 1.48±0.19 | 3.05±0.27* |
| Tg/HDL | 1.17±0.13 | 3.18±0.55* | 3.81±0.58* | 1.91±0.31 | 3.57±0.49* |
| bSBP, mmHg | 112±2 | 119±2 | 128±2*# | 115±2 | 126±2* |
| bPP, mmHg | 40±2 | 43±2 | 49±2* | 42±1 | 47±2 |
| cSBP, mmHg | 103±2 | 109±2 | 117±2*# | 105±2 | 116±2* |
| cPP, mmHg | 31±2 | 33±2 | 37±2* | 31±1 | 37±2* |
| cIMT, mm | 0.61±0.02 | 0.67±0.03 | 0.69±0.02* | 0.62±0.02 | 0.70±0.02* |
| CDd, mm | 0.57±0.01 | 0.57±0.01 | 0.61±0.01*# | 0.57±0.01 | 0.60±0.01* |
| cCSA mm2 | 10.5±0.3 | 11.4±0.5 | 12.7±0.5* | 10.7±0.3 | 12.6±0.4* |
| PWVcf, m/s | 6.8±0.26 | 7.3±0.3 | 7.9±0.25* | 6.8±0.19 | 8.0±0.23* |
| HTN,% | 3 | 28 | 54*# | 8 | 54* |
| EF. % | 63±1 | 59±1 | 60±1 | 62±1 | 60±1 |
| Medications | |||||
| Diuretics, % | 1 (3) | 0 (0) | 5 (12) | 3 (6) | 7 (14) |
| B-blockers, % | 0 (0) | 2 (8) | 5 (12) | 2 (4) | 5 (10) |
| ACEI/ARB, % | 0 (0) | 1 (4) | 8 (19)*# | 0 (0) | 9 (18)* |
| Ca2+ blockers, % | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Statins, % | 2 (6) | 1 (4) | 7 (16) | 3 (6) | 7 (14) |
BMI: body mass index = normal BMI <25 kg/m2, overweight BMI 25-29.9 kg/m2, obese BMI>30 kg/m2; WC: waist circumference = Non-risk group, male< 102 cm, female <88 cm; at-risk group, male≥ 102 cm, female ≥88 cm
Abbreviations: b = brachial; c = central; HDL: high density lipoprotein; Tg: triglycerides; HOMA-IR: homeostatic model assessment Insulin Resistance; cIMT, carotid intima medial thickness; cCSA, common carotid cross sectional area; CDd, carotid diameter in diastole; PWVcf, carotid-femoral pulse wave velocity; SBP, systolic pressure; PP, pulse pressure; HTN: hypertension status; EF; ejection fraction; ACEI: angiotensin-converting enzyme inhibitors; ARB: angiotensin II receptor blockers; Ca2+: calcium channel blockers. For BMI groups
p<0.05 vs normal,
p<0.05 vs. overweight; for WC group,
p<0.05 vs. non-risk.
Relationship between obesity, blood pressure, and arterial structure/function
BMI group comparisons indicated that obese subjects had increased BP (brachial and central), cIMT, cCSA, CDd, and PWVcf vs. normal or overweight subjects (Table 1). However, AGI did not differ in obese compared to normal (23+3%) and overweight (23+3%) subjects. Similar findings were also evident when comparing BP and arterial parameters between WC groups (Table 1 and Supplemental Table 1).
Since obese subjects had a greater prevalence of CV risk factors, which may be responsible for the physiological differences, we examined the relationship between BMI (as a continuous variable) and markers of arterial structure and function after adjusting for CV risk factors. In stepwise backward elimination models, BMI remained a predictor for cCSA (β=0.39, p<0.01), CDd (β=0.40, p<0.01), bSBP (β=0.32, p<0.01), MAP (β=0.28, p<0.05), cSBP (β=0.22, p<0.05), AGI (β=−0.26 p<0.01) and AGI@HR75 (β=−0.23, p<0.01). The introduction of CV risk factors removed BMI as a predictor of bPP, cPP, PWVcf and cIMT leaving only age, Tg, and HTN status in the final models (Table 2). A similar relationship was found between WC (as a continuous variable) and cIMT, cCSA, CDd, cfPWV, AGI, and AGI@HR75 (Table 2). While WC remained a predictor of bSBP after adding CV risk factors to the regression models, the relationship between WC and cSBP was no longer evident; rather age and HTN status were the main predictors (Table 2). As for %BF the only differences noted compared to BMI or WC, was that %BF was not a predictor of AP, AGI or AGI@HR75. With the exception of AGI and AGI@HR75, no sex-by-BMI (or WC) interactions were evident in the final models. A significant sex-by-WC interaction term was noted for AGI and AGI@HR75 whereby AGI (or AGI@HR75) decreased in women but increase in men with increasing WC. Further, no sex-by-%BF interactions were noted. When HOMA-IR, HDL or the Tg/HDL ratio replaced glucose, insulin or Tg in the backward elimination models, the main findings were not changed (Supplemental Table 3).
Table 2.
Final backward elimination models of arterial structure and function
| cIMT | cCSA | CDd | PWVcf | bSBP | bPP | MAP | cSBP | cPP | AP | AGI | AGI@HR75 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI | -- | -- | 0.39 | <0.01 | 0.40 | <0.01 | -- | -- | 0.32 | <0.01 | -- | -- | 0.28 | 0.02 | 0.22 | 0.03 | -- | -- | −0.17 | 0.04 | −0.26 | <0.01 | −0.23 | <0.01 |
| Age | 0.16 | <0.01 | 0.39 | <0.01 | -- | -- | 0.45 | <0.01 | 0.20 | 0.02 | 0.39 | <0.01 | 0.34 | <0.01 | 0.55 | <0.01 | 0.58 | <0.01 | 0.52 | <0.01 | 0.48 | <0.01 | ||
| Sex | -- | -- | −0.21 | 0.04 | −042 | <0.01 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 0.25 | <0.01 | 0.35 | <0.01 | 0.43 | <0.01 |
| TG | 0.28 | <0.01 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Glucose | -- | -- | -- | -- | -- | -- | -- | -- | 0.26 | <0.01 | -- | -- | 0.24 | 0.02 | 0.21 | 0.02 | -- | -- | -- | -- | -- | -- | -- | -- |
| Insulin | -- | -- | -- | -- | -- | -- | -- | -- | −0.23 | 0.03 | -- | -- | −0.24 | 0.04 | −0.19 | 0.07 | -- | -- | -- | -- | -- | -- | -- | -- |
| HTN | -- | -- | -- | -- | -- | -- | 0.35 | <0.01 | 0.37 | <0.01 | 0.24 | 0.02 | 0.40 | <0.01 | 0.39 | <0.01 | 0.21 | 0.02 | 0.25 | <0.01 | 0.19 | 0.04 | 0.18 | 0.04 |
|
| ||||||||||||||||||||||||
| WC | -- | -- | 0.34 | <0.01 | 0.44 | <0.01 | -- | -- | 0.25 | 0.02 | -- | -- | 0.25 | 0.04 | -- | -- | -- | -- | −0.20 | 0.02 | −0.27 | <0.01 | −0.23 | <0.01 |
| Age | 0.43 | <0.01 | 0.29 | <0.01 | -- | -- | 0.45 | <0.01 | 0.18 | 0.04 | 0.39 | <0.01 | -- | -- | 0.30 | <0.01 | 0.55 | <0.01 | 0.60 | <0.01 | 0.55 | <0.01 | 0.51 | <0.01 |
| Sex | -- | -- | -- | -- | −0.33 | <0.01 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 0.21 | <0.01 | 0.28 | <0.01 | 0.37 | <0.01 |
| TG | 0.33 | <0.01 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Glucose | -- | -- | -- | -- | -- | -- | -- | -- | 0.26 | <0.01 | -- | -- | 0.24 | 0.02 | 0.19 | 0.03 | -- | -- | -- | -- | -- | -- | -- | -- |
| Insulin | -- | -- | -- | -- | -- | -- | -- | -- | −0.20 | 0.07 | -- | -- | −0.22 | 0.06 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| HTN | -- | -- | -- | -- | -- | -- | 0.35 | <0.01 | 0.38 | <0.01 | 0.24 | 0.02 | 0.40 | <0.01 | 0.43 | <0.01 | 0.21 | 0.02 | 0.27 | <0.01 | 0.19 | 0.04 | 0.18 | 0.04 |
|
| ||||||||||||||||||||||||
| %BF | -- | -- | -- | -- | 0.31 | 0.05 | -- | -- | 0.21 | 0.03 | -- | -- | 0.21 | 0.04 | 0.17 | 0.08 | -- | -- | -- | -- | -- | -- | -- | -- |
| Age | .041 | <0.01 | 0.18 | 0.09 | -- | -- | 0.45 | <0.01 | -- | -- | 0.34 | <0.01 | -- | -- | 0.23 | 0.01 | 0.55 | <0.01 | 0.59 | <0.01 | 0.55 | <0.01 | 0.52 | <0.01 |
| Sex | -- | -- | -- | -- | −0.53 | <0.01 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 0.25 | <0.01 | 0.32 | <0.01 | 0.41 | <0.01 |
| TG | 0.34 | <0.01 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | .188 | 0.07 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Glucose | -- | -- | 0.24 | 0.03 | 0.17 | 0.09 | -- | -- | 0.39 | <0.01 | 0.21 | 0.05 | .285 | <0.01 | 0.31 | <0.01 | -- | -- | -- | -- | -- | -- | -- | -- |
| Insulin | -- | -- | -- | -- | -- | -- | −0.18 | 0.09 | -- | -- | −.235 | 0.04 | −0.17 | 0.09 | -- | -- | -- | -- | -- | -- | -- | -- | ||
| HTN | -- | -- | 0.26 | 0.02 | -- | -- | 0.35 | <0.01 | 0.43 | <0.01 | 0.18 | 0.08 | .365 | <0.01 | 0.42 | <0.01 | 0.20 | 0.03 | 0.16 | 0.05 | -- | -- | -- | -- |
Abbreviations: b = brachial; c = central; BMI, body mass index; HDL, high density lipoprotein; HTN, hypertension; WC, waist circumference; TG, triglycerides; cIMT, carotid intima medial thickness; cCSA, common carotid cross sectional area; CDd, carotid diameter in diastole; PWVcf, carotid-femoral pulse wave velocity; HR, heart rate; SBP, systolic pressure; DBP, diastolic pressure; PP, pulse pressure; MAP, mean arterial pressure; AP, aortic augmentation pressure; AGI, aortic augmentation index; AGI@75HR, aortic augmentation adjusted for HR. Significance defined as p<0.05.
Relationship between obesity and resting left ventricular diastolic function
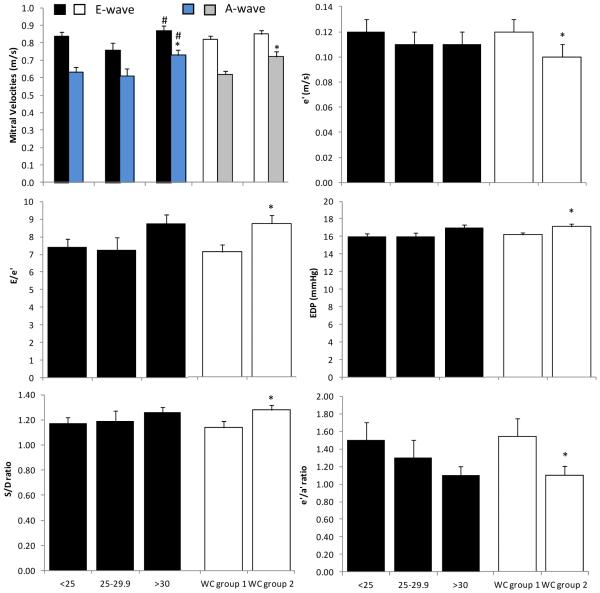
When comparing BMI groups, only the A-wave velocity was higher in obese vs. normal weight subjects (16%, p<0.05), whereas the E-wave velocity was higher in overweight vs. obese subjects (14%, p<0.05) (Figure 2). In contrast, WC group 2 had higher (p<0.05) values of A-wave, E/e’, EDP, S/D, and lower values of e’, and e’/a’ vs. WC group 1 (Figure 1, and Supplemental Table 2).
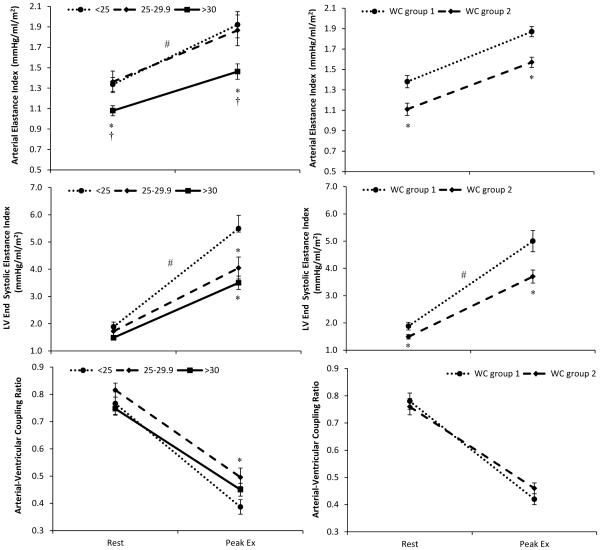
Figure 2. Arterial-ventricular coupling response to exercise.
The change from rest to peak exercise (Ex) in (A and B) effective arterial-elastance index, (C and D) left ventricular end-systolic elastance index, and (E and F) arterial-ventricular coupling ratio by body mass index (BMI: normal weight<25 kg/m2; overweight 25.0-29.9 kg/m2; and obese as BMI≥30 kg/m2) and waist circumference (WC: group 1 = non-risk male<102, female<88; group 2 = at risk-male≥ 102, female≥88) group. A group (BMI categories) by time (rest and peak Ex) interaction was found for EaI, and EesI whereby obese subjects had a blunted increase in EaI and EesI during exercise. Further, overweight subjects also had a blunted increase in EesI vs. normal weight subjects during exercise. As for the relationship between WC and arterial-ventricular coupling, a significant group (WC) by time (rest and peak Ex) interaction was found for EesI, but not for EaI or EaI/EesI. *p<0.05 compared to normal weight subjects (i.e., BMI<25 kg/m2, or WC group 1) † vs. overweight subjects; #p<0.05 time by group interaction. Data presented as means ± SEM.
Figure 1. Effects of obesity on left ventricular diastolic function.
Resting (A) mitral early (E-wave) and late (A-wave) velocities; (B) early tissue Doppler (e’) diastolic velocity of the lateral mitral annulus; (C) ratio of E-wave to e’ (E/e’); (D) end diastolic pressure (EDP); (E) ratio of peak systolic (S) velocity to peak anterograde diastolic (D) velocity of the pulmonary venous flow (S/D); and (F) the ratio of e’ to late tissue Doppler (a’) diastolic velocity (e’/a’) by body mass index (BMI: normal weight<25 kg/m2; overweight 25.0-29.9 kg/m2; and obese as BMI ≥30 kg/m2) or waist circumference (WC: group 1 = non-risk male<102, female<88; group 2 = at-risk male≥102, female≥88) groups. BMI was related to A-wave, whereas WC was related to most of the markers of diastolic function. *p<0.05 compared to normal weight subjects (i.e., BMI<25 kg/m2, or WC group 1) # vs. overweight subjects. Data presented as means ± SEM.
When examining the backward elimination regression models adjusting for the CV risk factors, BMI predicted only A-wave velocity (β=0.56, p<0.01), S/D (β=0.23, p=0.03), and e’/a’ (β=−0.21, p=0.04). Whereas for E-wave, E/A ratio, e’, E/e’ and EDP, age was the dominate predictor, not BMI (Table 3). In contrast, WC remained a predictor of A-wave (β=0.21, p=0.03), E/e’ (β=0.28, p<0.01), EDP (β=0.28, p<0.01), S/D (β=0.24, p=0.03) and e’/a’ (β=−0.27, p<0.01) in the regression models (Table 3). Of note, %BF provided similar findings to that between WC and diastolic function. When HOMA-IR, HDL or the Tg/HDL ratio replaced Tg in the regression models, minor differences were found which again did not alter the relationships (Supplemental Table 3). The only differences noted were that HOMA-IR, and age, was associated with e’ in the WC models, whereas in the BMI models, HOMA-IR, along with age, was associated with e’, and E/e’.
Table 3.
Final backward elimination models of left ventricular diastolic function
| E-wave | A-wave | E/A | e` | E/e` | EDP | S/D | e`/a` | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | Sig. | β | Sig. | β | Sig. | β | Sig. | β | Sig. | β | Sig. | β | Sig. | β | Sig. | |
| BMI | -- | -- | 0.30 | <0.01 | -- | -- | -- | -- | -- | -- | -- | -- | 0.23 | 0.03 | −0.21 | 0.04 |
| Age | −0.24 | 0.03 | 0.56 | <0.01 | −0.67 | <0.01 | -- | -- | 0.43 | <0.01 | 0.36 | <0.01 | 0.48 | <0.01 | −0.47 | <0.01 |
| Sex | 0.36 | <0.01 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | −0.34 | <0.01 | -- | -- |
| TG | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | −0.34 | <0.01 | -- | -- |
| Glucose | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Insulin | -- | -- | -- | -- | -- | -- | -- | -- | 0.28 | <0.01 | 0.31 | <0.01 | -- | -- | -- | -- |
| HTN | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
|
| ||||||||||||||||
| WC | -- | -- | 0.21 | 0.03 | -- | -- | -- | -- | 0.28 | <0.01 | 0.28 | <0.01 | 0.24 | 0.03 | −0.27 | <0.01 |
| age | −0.24 | 0.04 | 0.56 | <0.01 | −0.67 | <0.01 | −0.61 | <0.01 | 0.42 | <0.01 | 0.36 | <0.01 | 0.45 | <0.01 | −0.46 | <0.01 |
| Sex | 0.36 | <0.01 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | −0.29 | <0.01 | -- | -- |
| TG | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | −0.35 | <0.01 | -- | -- |
| Glucose | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Insulin | -- | -- | -- | -- | -- | -- | −0.22 | 0.01 | -- | -- | 0.31 | <0.01 | -- | -- | -- | -- |
| HTN | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
|
| ||||||||||||||||
| %BF | 0.21 | 0.09 | 0.33 | <0.01 | -- | -- | -- | -- | 0.23 | 0.04 | 0.23 | 0.04 | -- | -- | −0.27 | 0.01 |
| Age | −0.29 | 0.01 | 0.46 | <0.01 | −0.67 | <0.01 | −0.61 | <0.01 | 0.37 | <0.01 | 0.37 | <0.01 | −0.45 | <0.01 | −0.38 | <0.01 |
| Sex | 0.28 | 0.02 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| TG | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Glucose | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Insulin | -- | -- | -- | -- | -- | -- | −0.22 | 0.01 | 0.20 | 0.07 | 0.20 | 0.07 | -- | -- | -- | -- |
| HTN | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
Abbreviations: BMI, body mass index; HDL, high density lipoprotein; HTN, hypertension; WC, waist circumference; TG, triglycerides; E-wave, peak velocity of the early diastolic mitral flow; A-wave, peak velocity of the late diastolic mitral flow; e’, mitral annular early diastolic velocity; EDP, end diastolic pressure; S, peak systolic velocity; D, peak anterograde diastolic velocity; a’, mitral annular late diastolic velocity. Significance defined as p<0.05.
Relationship between obesity and resting and peak exercise arterial ventricular coupling and aerobic capacity
At rest, both EaI (p<0.05) and EesI (p=0.06) were reduced in obese compared to normal weight subjects (19-22%), and EaI was lower in obese vs. overweight (23%, p<0.05) subjects (Figure 2). As such, the EaI/EesI ratio did not differ between BMI groups at rest (Figure 2). At peak exercise, obese subjects also had a reduced (p<0.05) EaI compared to normal (27%) and overweight (23%) subjects. Similarly, obese subjects had a reduced peak EesI compared to normal weight subjects (39%, p<0.05), and the overweight subjects had a reduced peak EesI compared to normal weight subjects (28%, p<0.05). No differences were found between obese and overweight subjects for peak EesI. As such peak EaI/EesI did not differ by BMI group. Obese subjects also had a reduced VO2peak compared to normal weight subjects (16.5±0.7 vs. 23.3±1.2 ml/kg/min, p<0.05) but not the overweight subjects (16.5±0.7 vs. 19.9±1.5 ml/kg/min, p>0.05). A similar story was found when comparing WC groups for both resting and peak exercise, EaI, EesI, EaI/EesI, and VO2peak (Supplemental Table 2). A significant group (BMI categories) by time (rest vs. peak Ex) interaction was found for EaI, and EesI whereby obese subjects demonstrated a blunted increase in EaI and EesI during exercise (Figure 2). Overweight subjects also had a blunted increase in EesI during exercise compared to normal weight subjects. However, the group by time interaction for EaI/EesI did not reach statistical significance (p=0.06). As for the relationship between WC and arterial-ventricular coupling, a significant group by time interaction was found for EesI, but not for EaI or EaI/EesI (Figure 2).
In the regression models, BMI remained a significant predictor of resting EaI (β=−0.32, p<0.01), and EesI (β=−0.36, p<0.01) but not for resting EaI/EesI. Similarly, at peak exercise, BMI remained a significant predictor of EaI (β=−0.29, p<0.05), and EesI (β=−0.33, p<0.01) but not EaI/EesI. Further, BMI remained a predictor of VO2peak (β=−0.36, p<0.01) (Table 4). When BMI was replaced with WC, a similar story was evident in that at rest and peak exercise WC remained in the backward elimination models for both EaI and EesI, and VO2peak, but not for resting or peak exercise EaI/EesI. However, unlike BMI or WC, no relationship existed between resting and peak exercise EaI and EesI and %BF. For peak EesI this was attributed in part to a sex-by%BF interaction (p=0.058) whereby EesIpeak decreased with increasing %BF, but in men there was no relationship between peak EesI and %BF. A significant sex-by-WC interaction was also noted for peak EesI whereby slope of the decrease in peak EesI was stronger in women than men. Of note, no sex-by-BMI interactions were noted. When HOMA-IR, HDL or the Tg/HDL ratio replaced Tg in the backward elimination models, minor differences were found which did not interfere with how WC or BMI predicted arterial-ventricular coupling or VO2peak (Supplemental Table 3).
Table 4.
Final backward elimination models of resting and peak exercise arterial-ventricular coupling and aerobic capacity
| Rest EaI | Peak EaI | Rest EesI | Peak EesI | Rest EaI/EesI | Peak EaI/EesI | VO2peak | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | Sig. | β | Sig. | β | Sig. | β | Sig. | β | Sig. | β | Sig. | β | Sig. | |
| BMI | −0.33 | <0.01 | −0.36 | <0.01 | −0.28 | 0.01 | −0.33 | <0.01 | -- | -- | -- | -- | −0.36 | <0.01 |
| Age | -- | -- | -- | -- | 0.19 | 0.05 | 0.18 | 0.07 | -- | -- | -- | -- | −0.31 | <0.01 |
| Sex | 0.26 | 0.02 | 0.41 | <0.01 | 0.28 | <0.01 | 0.31 | <0.01 | −0.38 | <0.01 | -- | -- | −0.30 | <0.01 |
| TG | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 0.34 | <0.01 | -- | -- |
| Glucose | 0.22 | 0.06 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Insulin | -- | -- | −0.25 | 0.05 | −0.32 | <0.01 | −0.23 | 0.05 | -- | -- | -- | -- | -- | -- |
| HTN | -- | -- | 0.28 | 0.02 | -- | -- | -- | -- | -- | -- | -- | -- | −0.18 | 0.08 |
|
| ||||||||||||||
| WC | −0.59 | <0.01 | −0.51 | <0.01 | −0.30 | 0.02 | −0.36 | <0.01 | -- | -- | -- | -- | −0.33 | <0.01 |
| Age | -- | -- | 0.22 | 0.03 | 0.26 | 0.01 | -- | -- | -- | -- | −0.28 | <0.01 | ||
| Sex | -- | -- | 0.26 | 0.01 | 0.22 | 0.04 | 0.18 | 0.08 | −0.38 | <0.01 | -- | -- | −0.38 | <0.01 |
| TG | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 0.34 | <0.01 | -- | -- |
| Glucose | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Insulin | -- | -- | -- | -- | −0.30 | 0.01 | −0.22 | 0.07 | -- | -- | -- | -- | -- | -- |
| HTN | 0.24 | 0.05 | 0.32 | <0.01 | -- | -- | 0.26 | 0.01 | -- | -- | -- | -- | −0.20 | 0.06 |
|
| ||||||||||||||
| %BF | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | −0.49 | <0.01 |
| Age | -- | -- | 0.23 | 0.03 | -- | -- | 0.28 | 0.01 | -- | -- | -- | -- | −0.25 | 0.01 |
| Sex | 0.28 | 0.01 | 0.33 | <0.01 | 0.41 | <0.01 | 0.30 | <0.01 | −0.38 | <0.01 | -- | -- | -- | -- |
| TG | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 0.34 | <0.01 | -- | -- |
| Glucose | 0.21 | 0.08 | -- | -- | 0.23 | 0.05 | -- | -- | -- | -- | -- | -- | -- | -- |
| Insulin | −0.39 | <0.01 | −0.43 | <0.01 | −0.33 | <0.01 | −0.28 | 0.01 | -- | -- | -- | -- | -- | -- |
| HTN | -- | -- | -- | -- | -- | -- | −0.26 | 0.03 | -- | -- | -- | -- | −0.17 | 0.06 |
Abbreviations: BMI, body mass index; HDL, high density lipoprotein; HTN, hypertension; WC, waist circumference; TG, triglycerides; EaI, arterial elastance index; EesI, end systolic elastance index; EaI/EesI, arterial ventricular coupling ratio; VO2peak, peak oxygen consumption. Significance defined as p<0.05.
Discussion
A novel aspect of the present study is the simultaneous measurement of a number of anthropometric and CV (at rest and peak exercise) related indices in obese individuals without T2DM and overt CV disease. Our data suggests that obesity is reflected by carotid remodeling, increased central and brachial pressures, reduced resting LV diastolic function, along with decreased peak exercise LV contractility and aerobic capacity. Furthermore, WC may represent a better predictor of diastolic function than BMI. Importantly, these associations were independent of known CV risk factors, suggesting that pathophysiological changes manifest in obese individuals prior to evidence of chronic disease.
Obesity, blood pressures and arterial structure/function
HTN is approximately twice as prevalent in obese compared to normal weight individuals25. In addition, body fat distribution is thought to be an important contributor to the association between obesity and HTN26, 27. As such, bSBP has been shown to increase with increasing BMI and WC28-30. However, the relationship between BMI, WC, %BF and central pressures in individuals without overt CV disease or T2DM has not been well examined. In the present study, overall adiposity rather than central adiposity plays a significant role in increasing both bSBP and cSBP. Our results confirm and expand on the findings by Kolade et al.29 who identified a differential association between bSBP and cSBP with WC/hip ratio. It has been postulated29 that the increased HR, cardiac output and peripheral vasodilatation identified in “healthy” obese subjects31, 32 may explain the differences between bSBP and cSBP and body size. Our data suggests that cardiac output and peripheral resistance, but not HR, play a role in the differential association between bSBP and cSBP and body size.
Although, arterial stiffness and cIMT has been shown to be greater in obese individuals4, 33, this increased arterial remodeling likely reflects the co-occurrence of CV risk factors with obesity, specifically age and HTN. Whereby at higher distending pressures the force on the arterial wall is transferred to the stiffer collagen limiting distention. This suggests that PWVcf and cIMT may not be a pathophysiological consequence of obesity per se, but rather a reflection of the co-occurrence of CV risk factors in particular age, HTN, and Tg.
As previously reported29, 34 we found an inverse association with AP and AGI and both BMI and WC, which seems counterintuitive. Of note, this inverse relationship maybe sex dependent as AGI increased in men but decreased in women with increasing BMI or WC, however, further research is required to verify this sex difference. A potential mechanism as to why with increasing adiposity AGI is lower could be a reflection of the dilation of the central arteries. In our study, carotid lumen diameter increased with increasing BMI and WC. Similarly, an increase in radial artery diameter and compliance has been reported in obese individuals35. Such differences likely act to dampen the pressure amplification resulting in a reduced AGI. Indeed in our study, AP (a measure of the contribution of wave reflections to SBP) was inversely related to BMI and WC. Interestingly, %BF was not related to AP, or AGI. Whether, this arterial dilation and compliance is an initial adaptive response and that persistent exposure to an obese state or with the presence of overt CV disease, results in a reversal of this association remains unknown and requires further study.
Resting LV diastolic function
LV diastolic function is thought to be a pre-cursor of LV systolic dysfunction and heart failure 36-38, and a risk factor for all-cause and CV mortality37. This highlights the clinical importance of recognizing the early, and/or subclinical, differences in diastolic function. Increasing adiposity has been shown to be a predictor of resting LV diastolic function8, 39. However, we did not find strong differences between BMI groups for markers of diastolic function. In contrast, a number of markers of diastolic function differed by WC groups, and in the regression models. Although evidence has shown that general adiposity (by BMI) is a strong predictor of diastolic function8, 39, this may reflect a greater level of diastolic dysfunction (grade 1 and 2) due to the inclusion of individuals with T2DM, and overt CV disease. This would suggest that central adiposity maybe more sensitive to identifying the initial differences in diastolic function in individuals without overt CV disease, whereas, BMI may not be a predictor of diastolic dysfunction until after the onset of chronic CV disease. Further, WC seems to be a stronger predictor over BMI of myocardial infarction, ischemic stroke, and for total and CV mortality40, 41. Taken together these findings suggest a link between central adiposity and diastolic function as one of the underlying causes of an increased CV mortality risk.
Obesity and arterial-ventricular coupling
Arterial-ventricular coupling, and its determinants, are important for CV performance10 and a predictor of CV mortality11. With aging, in individuals with the metabolic syndrome, and in heart failure patients, resting EaI/EesI is maintained around 0.7-1.0 to optimize CV efficiency19, 42, 43. Indeed, with increasing BMI (WC or %BF) EaI/EesI remained stable at 0.75-0.82 at the expense of EaI and EesI which decreased with increasing BMI or WC, but not %BF. This differs from the findings of Chirinos et al.,21 who suggested that EesI and EaI increase with increasing BMI. However, these findings did not take into account the CV risk factors associated with increasing adiposity. Further, the decrease in EaI, a measure of net arterial load, may be explained in part by the lack of association between BMI (or WC) and PWVcf and the reduction in AGI with increasing BMI (or WC). The reduction in resting EesI may reflect a decrease in LV contractile function or simply a compensatory response to ensure that the coupling ratio is maintained within normal limits for optimal CV performance. To what extent this relationship remains evident after the development of T2DM or overt CV disease with increasing adiposity warrants further examination.
During exercise, EaI/EesI decreases due to an acute mismatch between EesI and EaI to optimize CV performance10. Although EaI/EesI decreased during exercise, no differences in peak EaI/EesI were identified with increasing obesity because of a similar decrease in peak EaI and EesI. We have previously shown that peak EaI/EesI is not maintained in individuals with the metabolic syndrome19, in which obesity is a key determinant. This would suggest that the added CV risk factors that determine metabolic syndrome (Tg, glucose, HTN) have a critical role in the decreased peak exercise EaI/EesI. The decrease in peak EesI with increasing adiposity likely reflects a reduction in peak exercise LV contractility, which also corresponded to a decrease in VO2peak with increasing adiposity.
Measures of obesity
We show that in the most part BMI, WC, and %BF provide similar insights into the CV dysfunction noted with obesity, with the exception of LV diastolic function in which WC and %BF seem to be better predictors. Further, %BF was not associated with some markers of arterial health (AP, AGI) and arterial-ventricular coupling. These differences may reflect how the obesity parameters represent different fat compartments, with BMI representing an estimation of total adiposity, and WC a measure of central visceral adiposity, sub-cutaneous adipose depots (or both)13, 14. However, BMI has poor sensitivity for calculating %BF13, and it does not take into account body fat distribution, which may be more important than total adiposity as a risk factor for CV disease. For example, pericardial and perivascular adipose tissue are not accounted for in these measures of obesity. These fat deposits are associated with impaired arterial and cardiac function, due to their direct effects on the tissue44, 45. More direct measures (magnetic resonance imaging or computed tomography) of fat distribution may provide greater insight into the role of site specific fat accumulation.
Obesity is a pro-inflammatory state46, 47, and pro-inflammatory cytokines play a role in both CV dysfunction48,49. In particular it has been postulated that intra-abdominal or visceral fat depots have the strongest impact on inflammatory markers50, which may, in part, explain the greater associations noted between WC and CV function compared to BMI or %BF. Further, sex-differences are noted in the association between adiposity and inflammation in particular the type/location of adiposity51, 52. Such differences may account for the sex-by-WC (or BMI) for AGI, and peak exercise EesI (for %BF). Further, research is required in this interersting topic.
Limitations
This was a cross-sectional study rather than a prospective study, and therefore we cannot identify causality. We are not able to exclude the influence of CV medications, which may have altered CV associations. However, hypertensive medications were used to identify the prevalence of HTN which was added into the regression models. Although pressure and flow were not directly measured, but rather estimated from non-invasive surrogates, these have been previously validated against invasive hemodynamic measurements performed at rest53, 54. Also, the non-invasive measurement of EesI has not been validated during exercise. Given the small number of men in our study, the sex differences noted should be regarded as preliminary, until we or others can obtain data on a larger population of male subjects. Finally, differences in the association between BMI, WC and %BF and CV function, may be due to the degree of inflammation that may differ depending on the pattern of fat distribution in the body. In conclusion, this study demonstrates that individuals with increasing obesity without T2DM and overt CV disease, display evidence of arterial remodeling, reduced resting LV diastolic and systolic function, along with an impaired peak exercise LV contractile function and exercise intolerance. Further with the exception of cSBP and LV diastolic function, both increasing BMI and WC (as markers of obesity) display similar levels of CV dysfunction.
Supplementary Material
Acknowledgements
The authors thank Charles Murray and Diana Stofcheck for their help with the echocardiography.
Sources of Funding
This study was supported in part by the American Heart Association 11CRP7370056 (Dr Chantler), National Heart, Lung, Blood Institute T32-HL090610 (Sara Fournier), and the National Institute of General Medical Sciences of the National Institutes of Health under Award Number U54GM104942. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Author Contributions
Evan DeVallance, Sara Fournier, Paul Chantler: conception, design, data analysis, drafting and revising of the manuscript
David Donley, Daniel Bonner, Kyuwan Lee, Jefferson Frisbee: data collection, interpretation of results, writing and reviewing of the manuscript
Conflict of Interest: The authors have nothing to disclose
Supplementary information is available at International Journal of Obesity's website
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of Obesity and Trends in Body Mass Index Among US Children and Adolescents, 1999-2010. Jama-J Am Med Assoc. 2012;307(5):483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kopelman P. Health risks associated with overweight and obesity. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2007;8(Suppl 1):13–7. doi: 10.1111/j.1467-789X.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- 3.Pascual M, Pascual DA, Soria F, Vicente T, Hernandez AM, Tebar FJ, et al. Effects of isolated obesity on systolic and diastolic left ventricular function. Heart. 2003;89(10):1152–6. doi: 10.1136/heart.89.10.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maher V, O'Dowd M, Carey M, Markham C, Byrne A, Hand E, et al. Association of central obesity with early Carotid intima-media thickening is independent of that from other risk factors. Int J Obes (Lond) 2009;33(1):136–43. doi: 10.1038/ijo.2008.254. [DOI] [PubMed] [Google Scholar]
- 5.Chirinos JA, Segers P, De Buyzere ML, Kronmal RA, Raja MW, De Bacquer D, et al. Left Ventricular Mass: Allometric Scaling, Normative Values, Effect of Obesity, and Prognostic Performance. Hypertension. 2010;56(1):91–98. doi: 10.1161/HYPERTENSIONAHA.110.150250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckel RH, York DA, Rossner S, Hubbard V, Caterson I, Jeor ST, St, et al. Prevention conference VII - Obesity, a Worldwide Epidemic Related to Heart Disease and Stroke - Executive summary. Circulation. 2004;110(18):2968–2975. doi: 10.1161/01.CIR.0000140086.88453.9A. [DOI] [PubMed] [Google Scholar]
- 7.Kannel WB, Cupples LA, Ramaswami R, Stokes J, Kreger BE, Higgins M. Regional Obesity and Risk of Cardiovascular-Disease - the Framingham-Study. J Clin Epidemiol. 1991;44(2):183–190. doi: 10.1016/0895-4356(91)90265-b. [DOI] [PubMed] [Google Scholar]
- 8.Canepa M, Strait JB, Abramov D, Milaneschi Y, AlGhatrif M, Moni M, et al. Contribution of central adiposity to left ventricular diastolic function (from the Baltimore Longitudinal Study of Aging) Am J Cardiol. 2012;109(8):1171–8. doi: 10.1016/j.amjcard.2011.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kenchaiah S, Evans JC, Levy D, Wilson PWF, Benjamin EJ, Larson MG, et al. Obesity and the Risk of Heart Failure. New England Journal of Medicine. 2002;347(5):305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 10.Chantler PD, Lakatta EG, Najjar SS. Arterial-ventricular coupling: mechanistic insights into cardiovascular performance at rest and during exercise. Journal of Applied Physiology. 2008;105(4):1342–1351. doi: 10.1152/japplphysiol.90600.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antonini-Canterin F, Enache R, Popescu BA, Popescu AC, Ginghina C, Leiballi E, et al. Prognostic value of ventricular-arterial coupling and B-type natriuretic peptide in patients after myocardial infarction: a five-year follow-up study. J Am Soc Echocardiogr. 2009;22(11):1239–45. doi: 10.1016/j.echo.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Borlaug BA, Kass DA. Ventricular-Vascular Interaction in Heart Failure. Heart Failure Clinics. 2008;4(1):23–36. doi: 10.1016/j.hfc.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Collazo-Clavell ML, Korinek J, et al. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes (Lond) 2008;32(6):959–66. doi: 10.1038/ijo.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han TS, McNeill G, Seidell JC, Lean ME. Predicting intra-abdominal fatness from anthropometric measures: the influence of stature. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 1997;21(7):587–93. doi: 10.1038/sj.ijo.0800446. [DOI] [PubMed] [Google Scholar]
- 15.Bigaard J, Tjonneland A, Thomsen BL, Overvad K, Heitmann BL, Sorensen TI. Waist circumference, BMI, smoking, and mortality in middle-aged men and women. Obesity research. 2003;11(7):895–903. doi: 10.1038/oby.2003.123. [DOI] [PubMed] [Google Scholar]
- 16.Janssen I, Heymsfield SB, Allison DB, Kotler DP, Ross R. Body mass index and waist circumference independently contribute to the prediction of nonabdominal, abdominal subcutaneous, and visceral fat. American Journal of Clinical Nutrition. 2002;75(4):683–688. doi: 10.1093/ajcn/75.4.683. [DOI] [PubMed] [Google Scholar]
- 17.Shah NR, Braverman ER. Measuring Adiposity in Patients: The Utility of Body Mass Index (BMI), Percent Body Fat, and Leptin. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0033308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donley DA, Fournier SB, Reger BL, DeVallance E, Bonner DE, Olfert IM, et al. Aerobic Exercise Training Reduces Arterial Stiffness in Metabolic Syndrome. Journal of Applied Physiology. 2014 doi: 10.1152/japplphysiol.00151.2014. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fournier SB, Reger BL, Donley DA, Bonner DE, Warden BE, Gharib W, et al. Exercise reveals impairments in left ventricular systolic function in patients with metabolic syndrome. Experimental Physiology. 2013;99(1):149–163. doi: 10.1113/expphysiol.2013.075796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoit BD. Left ventricular diastolic function. Crit Care Med. 2007;35(8 Suppl):S340–7. doi: 10.1097/01.CCM.0000270246.00349.F1. [DOI] [PubMed] [Google Scholar]
- 21.Chirinos JA, Rietzschel ER, De Buyzere ML, De Bacquer D, Gillebert TC, Gupta AK, et al. Arterial Load and Ventricular-Arterial Coupling. Hypertension. 2009;54(3):558–566. doi: 10.1161/HYPERTENSIONAHA.109.131870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chantler PD, Clements RE, Sharp L, George KP, Tan LB, Goldspink DF. The influence of body size on measurements of overall cardiac function. Am J Physiol Heart Circ Physiol. 2005;289(5):H2059–65. doi: 10.1152/ajpheart.00022.2005. [DOI] [PubMed] [Google Scholar]
- 23.Batterham AM, Vanderburgh PM, Mahar MT, Jackson AS. Modeling the influence of body size onV˙o 2 peak: effects of model choice and body composition. Journal of Applied Physiology. 1999;87(4):1317–1325. doi: 10.1152/jappl.1999.87.4.1317. [DOI] [PubMed] [Google Scholar]
- 24.National Institutes of Health NH, Lung, and Blood Institute Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obesity research. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 25.Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988-1994 and 1999-2004. Hypertension. 2008;52(5):818–27. doi: 10.1161/HYPERTENSIONAHA.108.113357. [DOI] [PubMed] [Google Scholar]
- 26.Johnson D, Prud'homme D, Despres JP, Nadeau A, Tremblay A, Bouchard C. Relation of abdominal obesity to hyperinsulinemia and high blood pressure in men. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 1992;16(11):881–90. [PubMed] [Google Scholar]
- 27.Okosun IS, Prewitt TE, Cooper RS. Abdominal obesity in the United States: prevalence and attributable risk of hypertension. J Hum Hypertens. 1999;13(7):425–30. doi: 10.1038/sj.jhh.1000862. [DOI] [PubMed] [Google Scholar]
- 28.Dyer AR, Elliott P. The INTERSALT study: relations of body mass index to blood pressure. INTERSALT Co-operative Research Group. J Hum Hypertens. 1989;3(5):299–308. [PubMed] [Google Scholar]
- 29.Kolade OO, O'Moore-Sullivan TM, Stowasser M, Coombes JS, Fassett RG, Marwick TH, et al. Arterial stiffness, central blood pressure and body size in health and disease. Int J Obes (Lond) 2012;36(1):93–9. doi: 10.1038/ijo.2011.79. [DOI] [PubMed] [Google Scholar]
- 30.Siani A, Cappuccio FP, Barba G, Trevisan M, Farinaro E, Iacone R, et al. The Relationship of Waist Circumference to Blood Pressure: The Olivetti Heart Study*. American Journal of Hypertension. 2002;15(9):780–786. doi: 10.1016/s0895-7061(02)02976-x. [DOI] [PubMed] [Google Scholar]
- 31.Emdin M, Gastaldelli A, Muscelli E, Macerata A, Natali A, Camastra S, et al. Hyperinsulinemia and autonomic nervous system dysfunction in obesity: effects of weight loss. Circulation. 2001;103(4):513–9. doi: 10.1161/01.cir.103.4.513. [DOI] [PubMed] [Google Scholar]
- 32.Hirsch J, Leibel RL, Mackintosh R, Aguirre A. Heart rate variability as a measure of autonomic function during weight change in humans. Am J Physiol. 1991;2616:R1418–23. doi: 10.1152/ajpregu.1991.261.6.R1418. Pt 2. [DOI] [PubMed] [Google Scholar]
- 33.Zebekakis PE, Nawrot T, Thijs L, Balkestein EJ, van der Heijden-Spek J, Van Bortel LM, et al. Obesity is associated with increased arterial stiffness from adolescence until old age. J Hypertens. 2005;23(10):1839–46. doi: 10.1097/01.hjh.0000179511.93889.e9. [DOI] [PubMed] [Google Scholar]
- 34.Maple-Brown LJ, Piers LS, O'Rourke MF, Celermajer DS, O'Dea K. Increased arterial stiffness in remote Indigenous Australians with high risk of cardiovascular disease. J Hypertens. 2007;25(3):585–91. doi: 10.1097/HJH.0b013e328011f766. [DOI] [PubMed] [Google Scholar]
- 35.Mangoni AA, Giannattasio C, Brunani A, Failla M, Colombo M, Bolla G, et al. Radial artery compliance in young, obese, normotensive subjects. Hypertension. 1995;266:984–8. doi: 10.1161/01.hyp.26.6.984. Pt 1. [DOI] [PubMed] [Google Scholar]
- 36.Schannwell CM, Schneppenheim M, Perings S, Plehn G, Strauer BE. Left ventricular diastolic dysfunction as an early manifestation of diabetic cardiomyopathy. Cardiology. 2002;98(1-2):33–9. doi: 10.1159/000064682. [DOI] [PubMed] [Google Scholar]
- 37.Bella JN, Palmieri V, Roman MJ, Liu JE, Welty TK, Lee ET, et al. Mitral ratio of peak early to late diastolic filling velocity as a predictor of mortality in middle-aged and elderly adults: the Strong Heart Study. Circulation. 2002;105(16):1928–33. doi: 10.1161/01.cir.0000015076.37047.d9. [DOI] [PubMed] [Google Scholar]
- 38.Kitzman DW, Gardin JM, Gottdiener JS, Arnold A, Boineau R, Aurigemma G, et al. Importance of heart failure with preserved systolic function in patients > or = 65 years of age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol. 2001;87(4):413–9. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 39.Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, et al. Effect of obesity and overweight on left ventricular diastolic function: a community-based study in an elderly cohort. J Am Coll Cardiol. 2011;57(12):1368–74. doi: 10.1016/j.jacc.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366(9497):1640–9. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 41.Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359(20):2105–20. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 42.Najjar SS, Schulman SP, Gerstenblith G, Fleg JL, Kass DA, O'Connor F, et al. Age and gender affect ventricular-vascular coupling during aerobic exercise. J Am Coll Cardiol. 2004;44(3):611–7. doi: 10.1016/j.jacc.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 43.Lam CS, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, et al. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation. 2007;115(15):1982–90. doi: 10.1161/CIRCULATIONAHA.106.659763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fox CS, Gona P, Hoffmann U, Porter SA, Salton CJ, Massaro JM, et al. Pericardial Fat, Intrathoracic Fat, and Measures of Left Ventricular Structure and Function: The Framingham Heart Study. Circulation. 2009;119(12):1586–1591. doi: 10.1161/CIRCULATIONAHA.108.828970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neeland IJ, Gupta S, Ayers CR, Turer AT, Rame JE, Das SR, et al. Relation of Regional Fat Distribution to Left Ventricular Structure and Function. Circulation: Cardiovascular Imaging. 2013;6(5):800–807. doi: 10.1161/CIRCIMAGING.113.000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19(4):972–8. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 47.Festa A, D'Agostino R, Jr., Williams K, Karter AJ, Mayer-Davis EJ, Tracy RP, et al. The relation of body fat mass and distribution to markers of chronic inflammation. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2001;25(10):1407–15. doi: 10.1038/sj.ijo.0801792. [DOI] [PubMed] [Google Scholar]
- 48.Fichtlscherer S, Rosenberger G, Walter DH, Breuer S, Dimmeler S, Zeiher AM. Elevated C-reactive protein levels and impaired endothelial vasoreactivity in patients with coronary artery disease. Circulation. 2000;102(9):1000–6. doi: 10.1161/01.cir.102.9.1000. [DOI] [PubMed] [Google Scholar]
- 49.Abramson JL, Weintraub WS, Vaccarino V. Association between pulse pressure and C-reactive protein among apparently healthy US adults. Hypertension. 2002;39(2):197–202. doi: 10.1161/hy0202.104270. [DOI] [PubMed] [Google Scholar]
- 50.Forouhi NG, Sattar N, McKeigue PM. Relation of C-reactive protein to body fat distribution and features of the metabolic syndrome in Europeans and South Asians. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2001;25(9):1327–31. doi: 10.1038/sj.ijo.0801723. [DOI] [PubMed] [Google Scholar]
- 51.Thorand B, Baumert J, Doring A, Herder C, Kolb H, Rathmann W, et al. Sex differences in the relation of body composition to markers of inflammation. Atherosclerosis. 2006;184(1):216–24. doi: 10.1016/j.atherosclerosis.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 52.Schreiner PJ, Terry JG, Evans GW, Hinson WH, Crouse JR, 3rd, Heiss G. Sex-specific associations of magnetic resonance imaging-derived intra-abdominal and subcutaneous fat areas with conventional anthropometric indices. The Atherosclerosis Risk in Communities Study. Am J Epidemiol. 1996;144(4):335–45. doi: 10.1093/oxfordjournals.aje.a008934. [DOI] [PubMed] [Google Scholar]
- 53.Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PA, et al. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol. 2001;38(7):2028–34. doi: 10.1016/s0735-1097(01)01651-5. [DOI] [PubMed] [Google Scholar]
- 54.Lee WS, Huang WP, Yu WC, Chiou KR, Ding PY, Chen CH. Estimation of preload recruitable stroke work relationship by a single-beat technique in humans. Am J Physiol Heart Circ Physiol. 2003;284(2):H744–50. doi: 10.1152/ajpheart.00455.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.