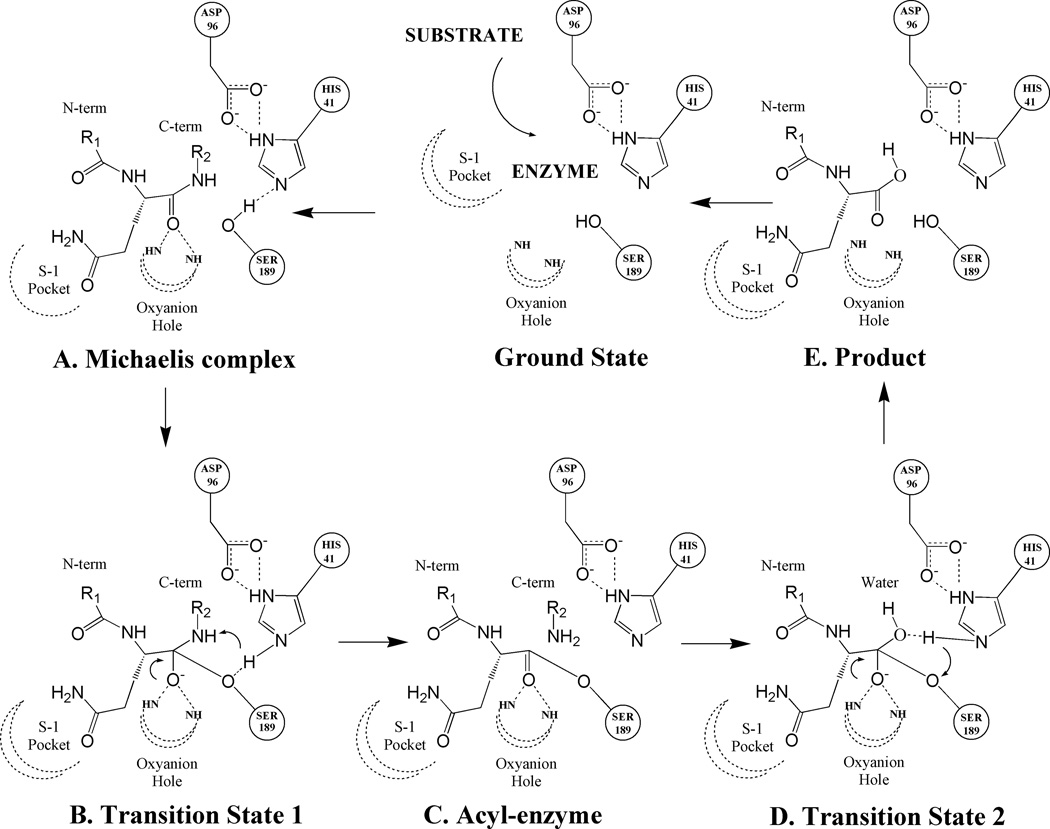
Figure 1.
Mechanistic scheme for the substrate hydrolysis reaction catalyzed by PSA. Illustrated in the scheme are catalytic triad residues (HIS41, SER189 and ASP96) and peptide substrate residue (with the P1 residue as glutamine) with its P1 residue docked at the catalytic site in the S-1 pocket. Dotted lines denote hydrogen bonds and curled arrows represent transfer of proton in a subsequent step. (A): Early non-covalent Michaelis complex (B): Initial tetrahedral transition state formed by the nucleophilic attack of catalytic serine residue (SER189) onto the carboxyl of the peptide bond being cleaved. (C): Acyl-enzyme complex formation between the substrate fragment on the N-terminal side of the cleaved peptide bond and the catalytic serine residue. (D): Second transition state formed upon the nucleophic attack of solvent water molecule onto the ester bond of acyl-enzyme complex. (E): Collapse of second transition state leads to the transfer of a water hydroxyl to the C-terminal carbon and a water proton to the catalytic serine residue. Ultimately, the product is released from the catalytic pocket and the enzyme returns to the ground state.