Key Points
PR-104 represents a potential novel treatment for relapsed/refractory T-ALL.
AKR1C3 expression could be used as a biomarker to select patients who may respond to PR-104 in prospective clinical trials.
Abstract
PR-104, a phosphate ester of the nitrogen mustard prodrug PR-104A, has shown evidence of efficacy in adult leukemia clinical trials. Originally designed to target hypoxic cells, PR-104A is independently activated by aldo-keto-reductase 1C3 (AKR1C3). The aim of this study was to test whether AKR1C3 is a predictive biomarker of in vivo PR-104 sensitivity. In a panel of 7 patient-derived pediatric acute lymphoblastic leukemia (ALL) xenografts, PR-104 showed significantly greater efficacy against T-lineage ALL (T-ALL) than B-cell-precursor ALL (BCP-ALL) xenografts. Single-agent PR-104 was more efficacious against T-ALL xenografts compared with a combination regimen of vincristine, dexamethasone, and l-asparaginase. Expression of AKR1C3 was significantly higher in T-ALL xenografts compared with BCP-ALL, and correlated with PR-104/PR-104A sensitivity in vivo and in vitro. Overexpression of AKR1C3 in a resistant BCP-ALL xenograft resulted in dramatic sensitization to PR-104 in vivo. Testing leukemic blasts from 11 patients confirmed that T-ALL cells were more sensitive than BCP-ALL to PR-104A in vitro, and that sensitivity correlated with AKR1C3 expression. Collectively, these results indicate that PR-104 shows promise as a novel therapy for relapsed/refractory T-ALL, and that AKR1C3 expression could be used as a biomarker to select patients most likely to benefit from such treatment in prospective clinical trials.
Introduction
Acute lymphoblastic leukemia (ALL) is the most common malignancy in children.1,2 Childhood ALL can be broadly divided into B-cell-precursor ALL (BCP-ALL, 80%-85% of cases) and T-lineage ALL (T-ALL, 15%-20%).3 Improvements in supportive care and use of multiagent chemotherapy have increased the overall survival for pediatric ALL to >90%,4 whereas adults and infants with ALL experience significantly inferior outcome, and biologically targeted therapy has had relatively little impact to date. Historically, patients with T-ALL have experienced a worse prognosis compared with their BCP-ALL counterparts, although contemporary regimens are now able to achieve cure rates comparable to those for patients with BCP-ALL.5 Despite this improvement in outcome, patients with T-ALL are more likely to experience induction therapy failure and early relapse.5 In addition, the outcome for patients with primary refractory and relapsed T-ALL remains dismal.5,6 Therefore, a continuing challenge for the management of T-ALL is to identify the most appropriate treatment of those patients who respond poorly to current treatment strategies.
The bone marrow microenvironment plays an important role in normal and malignant hematopoiesis. Hypoxic niches of the bone marrow microenvironment support hematopoiesis; indeed, some data suggest that hypoxia supports hematopoietic stem cells and leukemic blasts.7-12 The cells in the hypoxic niches of the bone marrow microenvironment are protected from chemotherapeutic drugs because of their low proliferation activity and potentially compromised drug delivery in poorly vascularized regions. These hypoxic niches are considered as important contributors to chemoresistance, and eventually to relapse.13,14
The identification of hypoxia as an important mediator of cell survival, resistance, and relapse in leukemia has prompted the development of compounds that are specifically activated under low oxygen tension, termed hypoxia-activated prodrugs (HAPs). Several HAPs have been evaluated in clinical trials,15 and TH-302 remains the most clinically advanced drug in this class.16,17 TH-302 is currently being investigated in over 19 clinical trials, with 2 trials studying its efficacy in hematologic malignancies (clinicaltrials.gov).
PR-104, like TH-302, is a prodrug of a nitrogen mustard18 which has shown some degree of efficacy in adult patients with relapsed/refractory acute myeloid leukemia (AML).19 PR-104 is a phosphate ester that is hydrolyzed in vivo to PR-104A, which is then metabolized under hypoxia by the 1-electron NADPH:cytochrome P450 oxidoreductase (CYPOR) and related flavoproteins to DNA cross-linking metabolites (PR-104H or PR-104M).20 Unlike TH-302, PR-104A is also activated to PR-104H independently of hypoxia by aldo-keto reductase 1C3 (AKR1C3).21 AKR1C3 is a member of a superfamily of NAD(P)H-linked oxidoreductases that reduce aldehydes and ketones to their corresponding primary and secondary alcohols.22 AKR1C3 plays important roles in steroid hormone and prostaglandin D2 metabolism, with a normal tissue distribution localized to the lung, liver, prostate, testis, and mammary glands.23 However, recent studies have shown that AKR1C3 is overexpressed in a number of human cancers including breast,24,25 prostate,26,27 and leukemia.28-30 AKR1C3-mediated prostaglandin D2 metabolism has been shown to regulate myeloid31 and erythroid28 differentiation, suggesting that AKR1C3 represents a novel target for the treatment of AML31 and chronic myelogenous leukemia.28 Taken together, it is proposed that PR-104 may be a targeted drug for cancers that either express high AKR1C3 or are hypoxic.
PR-104 has been shown to specifically target hypoxic regions of leukemia infiltration in preclinical models. Following IV inoculation, the NALM-6 ALL cell line caused extensive regions of hypoxia in the bone marrow of immune-deficient mice, as assessed by the specific chemical marker of hypoxia pimonidazole.32 Both pimonidazole staining and leukemia infiltration in the bone marrow were dramatically reduced following PR-104 administration. Moreover, PR-104 exhibited significant in vivo efficacy against pediatric T- and BCP-ALL patient-derived xenografts in immune-deficient mice when tested at its maximum tolerated dose (550 mg/kg).33 When tested at doses providing plasma pharmacokinetics achievable in humans (50-200 mg/kg in mice),34 PR-104 maintained its pronounced efficacy against a T-ALL xenograft, but not against a BCP-ALL xenograft.32 The mechanism for this differential sensitivity between T-ALL and BCP-ALL xenografts to PR-104 was not defined, however, it was hypothesized that the expression levels of AKR1C3 may determine sensitivity to PR-104 based on previously published data in solid tumors with high AKR1C3 expression.21 Overall, these findings suggest that PR-104 has the potential to target hematologic tumors with hypoxia and/or high AKR1C3 expression.
In this study, we used an extensive panel of patient-derived pediatric ALL xenografts to determine whether PR-104 exhibited lineage-specific in vivo efficacy at doses that provide pharmacokinetics achievable in human cancer patients, and to compare its single-agent efficacy with an induction-type regimen consisting of the established drugs vincristine, dexamethasone, and l-asparaginase (VXL).35 Moreover, we assessed the relationship between PR-104 in vivo efficacy or PR-104A in vitro efficacy against these xenografts and AKR1C3 messenger RNA (mRNA), protein, and activity levels. To demonstrate a causal relationship between AKR1C3 expression and sensitivity to PR-104/PR-104A, we overexpressed AKR1C3 in a resistant BCP-ALL xenograft and assessed its in vitro and in vivo responses. Finally, we tested whether primary patient T-ALL cells had increased ex vivo sensitivity to PR-104A compared with BCP-ALL cells. These results indicate that PR-104 represents a potential novel treatment for relapsed/refractory T-ALL and that AKR1C3 expression could be used as a biomarker to select patients who may respond to PR-104 in prospective clinical trials.
Materials and methods
Development of patient-derived xenografts and assessment of in vivo drug efficacy
All experimental studies were conducted with approvals from the Human Research Ethics Committee and the Animal Care and Ethics Committee of UNSW Australia (Sydney, Australia). Procedures by which we established continuous xenografts from childhood ALL biopsies in female nonobese diabetic/severe combined immunodeficient (NOD/SCID) (NOD.CB17-Prkdcscid/SzJ) mice or NOD/SCID/interleukin-2 receptor γ–negative (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ, NSG) mice (each of 20-25 g) and evaluated their in vivo sensitivity to PR-104 as a single agent or in combination with VXL in groups of 6-10 mice have been previously reported,33,35-39 and are described in detail in the supplemental Methods (available on the Blood Web site). PR-104 was supplied by Proacta Inc through the Cancer Therapy Evaluation Program of the National Cancer Institute, or by the Auckland Cancer Society Research Centre. Patient demographics and leukemia-specific details of samples that were used for xenografting are presented in Table 1. ALL subtypes were categorized at biopsy by their immunophenotype, and gene expression profiles of these xenografts have previously been published.40
Table 1.
Patient characteristics and clinical status for patient-derived xenografts
| Xenograft | Age at diagnosis, mo/sex | ALL subtype | Disease status at biopsy | Length of CR1, mo | Survival after relapse, mo | Current clinical status |
|---|---|---|---|---|---|---|
| ALL-2 | 65/F | c-ALL | Relapse 3 | 30 | 46 | DOD |
| ALL-3 | 154/F | Pre-B | Diagnosis | 38 | 212* | CR2 |
| ALL-4 | 105/M | Ph+ | Diagnosis | 10 | 1 | DOD |
| ALL-7 | 88/M | Biphen. | Diagnosis | 7 | 6 | DOD |
| ALL-8 | 152/M | T-ALL | Relapse 1 | 17 | 1 | DOD |
| ALL-10 | 48/M | c-ALL | Diagnosis | 138* | — | CR1 |
| ALL-11 | 37/F | c-ALL | Diagnosis | 204* | — | CR1 |
| ALL-16 | 122/F | T-ALL | Diagnosis | 63* | — | CR1 |
| ALL-17 | 107/F | c-ALL | Diagnosis | 25 | 165* | CR2 |
| ALL-19 | 194/M | c-ALL | Relapse 1 | 4 | 7 | DOD |
| ALL-27 | 104/M | T-ALL | Diagnosis | 10 | 19 | DOD |
| ALL-29 | 59/M | T-ALL | Diagnosis | 137* | — | CR1 |
| ALL-30 | 90/M | T-ALL | Diagnosis | 13 | — | DOD |
| ALL-31 | 123/M | T-ALL | Diagnosis | 5 | — | DOD |
| ALL-32 | 135/M | T-ALL | Relapse 1 | 10 | 68* | CR2 |
| ALL-33 | 89/M | T-ALL | Diagnosis | 73* | — | CR1 |
| ALL-42 | 32/M | T-ALL | Diagnosis | 16 | 5 | DOD |
Biphen, biphenotypic; c-ALL, common (CD10+) BCP-ALL; CR1, complete remission 1; CR2, complete remission 2; DOD, dead of disease; F, female; M, male; Pre-B, BCP-ALL.
No event (censored according to most recent clinic visit).
Immunoblotting
Procedures for the preparation of whole-cell protein extracts, determination of protein concentrations, and analysis of cellular proteins by immunoblotting have been described in detail elsewhere,41 and are detailed in the supplemental Methods.
RNA extraction and real-time quantitative reverse transcription–polymerase chain reaction
Total RNA was isolated from xenograft cells using the TRIzol (Invitrogen) method and purified with the RNeasy kit (Qiagen) according to the manufacturer’s instructions, and detailed in the supplemental Methods.
In vitro cell culture and cytotoxicity assays
Xenograft cells were retrieved from cryostorage and resuspended in QBSF-60 medium (Quality Biological) supplemented with 20 ng/mL Flt-3 ligand (Bionovus Life Sciences), 100 U/mL penicillin, 100 µg/mL streptomycin, and 2 mM l-glutamine. Viability was determined by exclusion of 0.2% trypan blue. For cytotoxicity experiments, cells were equilibrated in medium in a humidified atmosphere overnight at 37°C, 5% CO2 and then treated with PR-104A, daunorubicin, vincristine, dexamethasone, or l-asparaginase for 48 hours at which time cell viability was determined using a resazurin (Alamar blue) assay. Cell viability was calculated as a percentage of vehicle-treated controls. IC50 (the drug concentration that inhibited fluorescence to 50% of vehicle-treated control) values were calculated from cumulative survival curves.
Overexpression of AKR1C3 in a patient-derived xenograft
Development of the AKR1C3-transduced xenograft ALL-11, which expresses very low endogenous levels of AKR1C3, has been previously described.30 The transduced cells were inoculated into NSG mice for expansion. Following a first expansion, cells were sorted to purity using green fluorescent protein (GFP) as a reporter (FACSJazz and FACSAria; BD Biosciences) and inoculated into NSG mice for experiments.
Measurement of AKR1C3 enzymatic activity in xenograft cells using the fluorogenic probe coumberone
AKR1C3 enzymatic activity was measured following procedures described previously,30 and described in the supplemental Methods.
Ex vivo coculture of patient mononuclear cells and viability assay
Patient mononuclear cells were obtained from the Children’s Cancer Institute Tumor Bank having been collected from patients by iliac crest biopsy, purified by FICOL density gradient centrifugation, and cryopreserved in liquid nitrogen. Coculture assays were adapted from Suryani et al.40 MSC-hTERT cells were plated at 50 000 cells per well in a 96-well plate and cultured overnight in RPMI (Life Technologies) supplemented with 10% fetal calf serum. Patient cells were thawed, washed in RPMI medium, and plated at 100 000 cells per well in QBSF-60 medium supplemented with 20 ng/mL Flt-3 ligand, 100 U/mL penicillin, 100 µg/mL streptomycin, and 2 mM l-glutamine. Cells were cultured for 3 hours prior to addition of PR-104A in a serial dilution from 100 µM to 1.25 µM. Cells were cultured for 24 hours at 37°C in 5% CO2 prior to staining for viability. Viability was assessed with the 7-aminoactinomycin D (7AAD) vital stain (BD Biosciences) diluted in phosphate-buffered saline. Cells were incubated for 15 minutes, then filtered and analyzed on a FACSCalibur (BD Biosciences). MSC-hTERT cells are GFP-positive and were gated out. Quantitative analysis was performed using FlowJo vX software.
Statistical analysis
The Fisher exact test was performed to determine the efficacy of PR-104 treatment in BCP-ALL and T-ALL xenografts. A cutoff threshold was determined based on the mean value of the leukemia growth delay (LGD) for both groups. Based on this threshold, a contingency table was used to evaluate PR-104 sensitivity in BCP-ALL and T-ALL xenografts. Mantel-Cox survival analysis was used to evaluate the efficacy of PR-104 treatment in the animal study with AKR1C3 overexpression compared with empty vector control in ALL-11 xenografts. Correlations were analyzed with Spearman analysis. Group analysis with >2 groups and a normal distribution were analyzed by 1-way analysis of variance; if only 2 groups were compared an unpaired Student t test with Welch correction or a Mann-Whitney U test were used for normally and nonnormally distributed data, respectively.
Results
PR-104/PR-104A exhibited profound antileukemic efficacy against T-ALL xenografts in vivo and in vitro
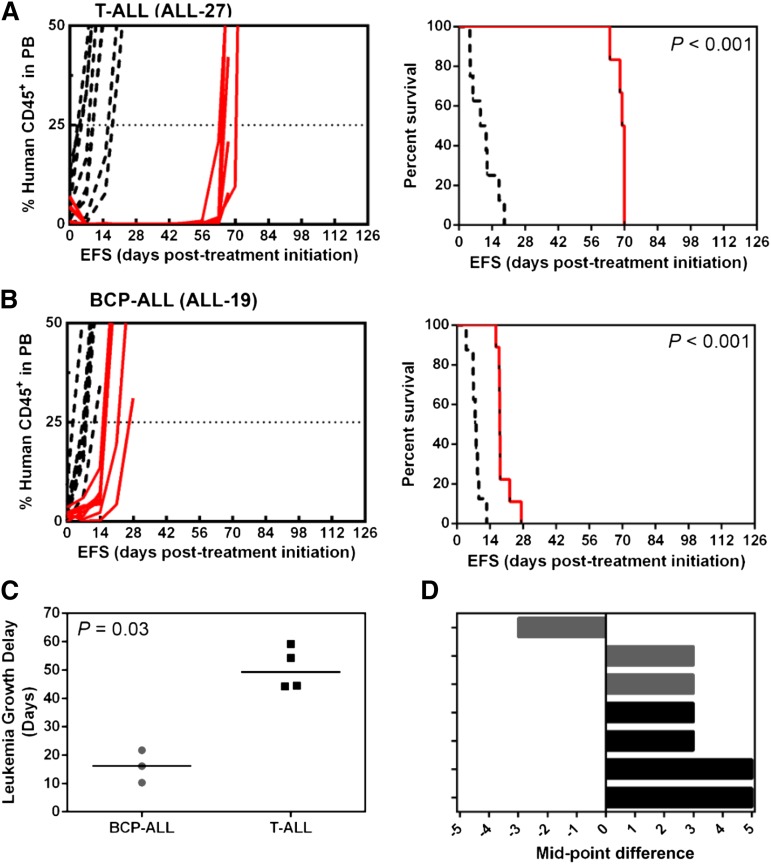
We previously observed preferential in vivo efficacy of PR-104 against a single T-ALL xenograft (ALL-8) compared with a BCP-ALL xenograft (ALL-19) when administered at clinically achievable doses.32,34 To evaluate whether PR-104 exhibited subtype-specific efficacy against T-ALL, we tested PR-104 against an extended panel of T-ALL (ALL-8, ALL-27, ALL-31, and ALL-29) and BCP-ALL (ALL-2, ALL-4, and ALL-19) xenografts, 6 of 7 of which were derived from patients who succumbed to their disease (Table 1). Two weekly doses of single-agent PR-104 at 200 mg/kg significantly delayed the progression of all 7 xenografts (Figure 1A-B; Table 2; supplemental Figure 1). LGDs ranged from 10.3 days (ALL-19) to 59.2 days (ALL-27) (Table 2) and was significantly greater for the 4 T-ALL xenografts than the 3 BCP-ALL xenografts (Fisher exact test, P = .03, Figure 1C). Using stringent objective response criteria,37 PR-104 induced objective responses in 4 of 4 T-ALL (2 complete responses [CRs]; 2 maintained CRs [MCRs]) and 2 of 3 BCP-ALL (2 CRs; 1 progressive disease 2 [PD2]) (Figure 1D; Table 2). A complete summary of results is provided in supplemental Table 2.
Figure 1.
In vivo responses to PR-104 of T-ALL and BCP-ALL engrafted mice. NOD/SCID mice were engrafted with a T-ALL, ALL-27 (A) or a BCP-ALL, ALL-19 (B) patient-derived xenograft. When the proportion of hCD45+ cells in the PB reached 1%, the mice were treated with PR-104 at 200 mg/kg weekly for 2 weeks (red), and compared with vehicle-treated control (black). When the proportion of hCD45 reached 25% in the PB, mice were scored as event on the corresponding Kaplan-Meier curves on the right panel. (C) LGD is plotted for the BCP-ALL and T-ALL xenografts. PR-104 showed a significantly greater efficacy in T-ALL than BCP-ALL xenografts (Fisher exact test, P = .03). (D) COMPARE-like plots show the objective response measures (ORMs) for 4 T-ALL (ALL-8, ALL-27, ALL-29, and ALL-31) (black) and 3 BCP-ALL (ALL-2, ALL-4, and ALL-19) (gray) xenografts. Responses represent the difference in xenograft objective response from the midpoint (0) representative of stable disease (SD). Bars to the right indicate an objective response (PR, CR, and MCR), whereas those to the left indicate PD. Data for the complete panel of xenografts are available in Table 2 and supplemental Figure 1.
Table 2.
Results of in vivo efficacy testing of PR-104 treatment at 200 mg/kg once a week for 2 weeks in T-ALL and BCP-ALL xenografts
| Subtype | Treatment | EFS, d | LGD, d | Median ORM | Overall response | |
|---|---|---|---|---|---|---|
| ALL-2 | BCP-ALL | Vehicle | 15.4 | |||
| PR-104 | 31.5 | 16.1 | 8 | CR | ||
| ALL-4 | BCP-ALL | Vehicle | 5.3 | |||
| PR-104 | 27 | 21.7 | 8 | CR | ||
| ALL-19 | BCP-ALL | Vehicle | 7.9 | |||
| PR-104 | 18.2 | 10.3 | 2 | PD2 | ||
| ALL-8 | T-ALL | Vehicle | 2.5 | |||
| PR-104 | 56.8 | 54.3 | 10 | MCR | ||
| ALL-27 | T-ALL | Vehicle | 10.3 | |||
| PR-104 | 69.5 | 59.2 | 10 | MCR | ||
| ALL-29 | T-ALL | Vehicle | 7.0 | |||
| PR-104 | 51.2 | 44.2 | 8 | CR | ||
| ALL-31 | T-ALL | Vehicle | 7.0 | |||
| PR-104 | 51.4 | 44.4 | 8 | CR |
P < 0.001 for the LGD of all xenografts treated with PR-104 compared with vehicle.
CR, complete response; EFS, event-free survival; MCR, maintained complete response; PD2, progressive disease 2.
To gain a greater perspective of the efficacy of PR-104 in relation to established drugs used to treat pediatric ALL, we combined PR-104 with an induction-type VXL regimen that was previously optimized in our laboratory35 against 3 T-ALL xenografts. VXL alone delayed the progression of the xenografts by 17.3 to 26.5 days and achieved 2 objective responses (1 partial response [PR]; 1 CR), compared with LGDs of 44.2 to 54.3 days and 3 of 3 objective responses (2 CRs, 1 MCR) for PR-104 as a single agent (supplemental Figure 2; supplemental Tables 1-2). The combination of VXL and PR-104 achieved LGDs of 34.5 to 49.5 days in the 3 xenografts, indicating that the combination was neither synergistic nor antagonistic, and also that PR-104 alone was just as effective. PR-104 was well tolerated both as a single agent and in combination with VXL, with no mice experiencing toxicity-related events (supplemental Table 2).
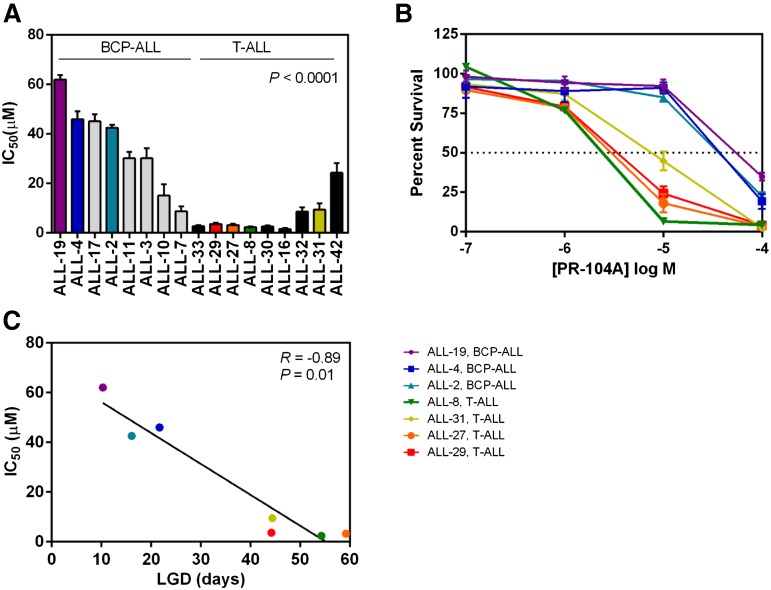
To assess whether the trend toward greater in vivo efficacy of PR-104 against T-ALL compared with BCP-ALL was representative of the larger panel of xenografts, we next assessed the in vitro sensitivity of 17 xenografts to PR-104A by resazurin assay. T-ALL xenografts (n = 9) were significantly more sensitive to PR-104A compared with BCP-ALL xenografts (n = 8, P < .0001, Figure 2A-B), with median IC50s of 3.1 µM and 36.3 µM, respectively. The in vitro PR-104A sensitivity (IC50s) of 7 xenografts showed a highly significant correlation (R = −0.89, P = .01) with their in vivo PR-104 responses (LGDs) (Figure 2C). These in vitro results indicate that the underlying mechanism(s) for differential in vivo PR-104 sensitivity between T-ALL and BCP-ALL xenografts are more likely to be cell intrinsic rather than due to possible differences in the in vivo hypoxic microenvironment.
Figure 2.
In vitro sensitivity to PR-104A in a panel of BCP-ALL and T-ALL xenografts. Xenograft cells were cultured for 48 hours in increasing concentrations of PR-104A (0.1-100 μM). Samples were then assayed with resazurin to determine viability based on mitochondrial activity. (A) The IC50 is plotted for the T-ALL and BCP-ALL xenograft panels. (B) Dose response curves of the viability of cells are shown at the different concentrations of PR-104A for the same xenografts that were used in vivo from Figure 1C-D. (C) In vitro and in vivo sensitivity to PR-104A showed a significant correlation in the 7 xenografts examined. Data represent the mean ± SEM of IC50 values for an individual xenograft from a minimum of 3 independent experiments.
AKR1C3 expression is a biomarker of in vivo/in vitro sensitivity to PR-104/PR-104A
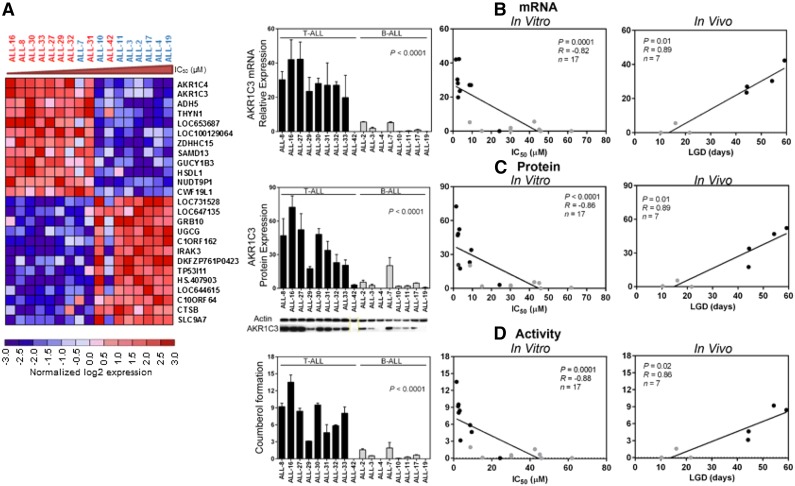
With the aim of identifying a biomarker for PR-104 sensitivity, we performed a microarray analysis of gene expression comparing PR-104A–sensitive and PR-104A–resistant xenografts using LIMMA. At a false discovery rate (FDR) <0.05, there were 370 upregulated and 295 downregulated genes associated with PR-104 sensitivity (FDR <0.05, P < .01) (supplemental Table 3). The top 2 differentially expressed genes were AKR1C4 and AKR1C3 (Figure 3A). Unlike AKR1C4, AKR1C3 has a known role in metabolism of PR-104A,21 indicating that AKR1C3 expression could account for the differential sensitivity of ALL xenografts to PR-104 and may be a useful biomarker.
Figure 3.
PR-104A sensitivity correlated with AKR1C3 mRNA, protein, and enzymatic activity. (A) A total of 17 ALL xenografts (T-ALL, n = 9; BCP-ALL, n = 8) were profiled on Illumina Human Ref 12 Beadchip arrays, and the top 25 differentially expressed genes between PR-104A–resistant and –sensitive xenografts; genes were ordered based on the P value. Red shows relative upregulation and blue shows relative downregulation. T-ALLs are shown in red and BCP-ALLs in blue. (B) mRNA expression of AKR1C3 by RT-qPCR in the panel of xenografts, and correlated with in vitro IC50 and in vivo LGD. (C) Protein lysates were extracted from T-ALL and BCP-ALL xenografts and AKR1C3 was probed by western blot, quantified, and correlated with IC50 and LGD. A representative immunoblot is shown; for all immunoblots, see supplemental Figure 3. (D) Through a SN34037-sensitive coumberone reduction, AKR1C3 enzymatic activity can be measured by the fluorescent product coumberol. Coumberol formation was measured in vitro in T-ALL and BCP-ALL xenografts, and correlated with in vitro IC50 and in vivo LGD.
The oxygen-insensitive reductase AKR1C3 is a known activator of PR-104A in solid tumor xenografts grown subcutaneously in mice.21 Therefore, we sought to determine whether differences in AKR1C3 mRNA expression, protein expression, and enzymatic activity could account for the greater sensitivity of T-ALL xenografts to PR-104 compared with BCP-ALL xenografts from the same panel of 17 xenografts. The AKR1C3 mRNA/protein expression and enzymatic activity were all significantly greater across the T-ALL compared with the BCP-ALL xenografts (Figure 3B-D; supplemental Figure 3). Moreover, all 3 parameters of AKR1C3 expression and activity strongly correlated with both in vitro sensitivity to PR-104A, and in vivo sensitivity to PR-104 (Figure 3B-D; supplemental Table 4). We have previously reported that preincubation with the AKR1C3-specific inhibitor SN34037 significantly decreased the in vitro PR-104A sensitivity of T-ALL, but not BCP-ALL, xenograft cells.30 We observed a strong correlation between the increase in PR-104A IC50 value and basal AKR1C3 protein expression levels across the entire panel of ALL xenografts (R = 0.86; P < .0001; supplemental Figure 4). These findings suggest that AKR1C3 is a major determinant of in vivo and in vitro sensitivity of ALL xenografts to PR-104 and PR-104A, respectively.
AKR1C3 expression confers sensitivity to PR-104 in a BCP-ALL xenograft
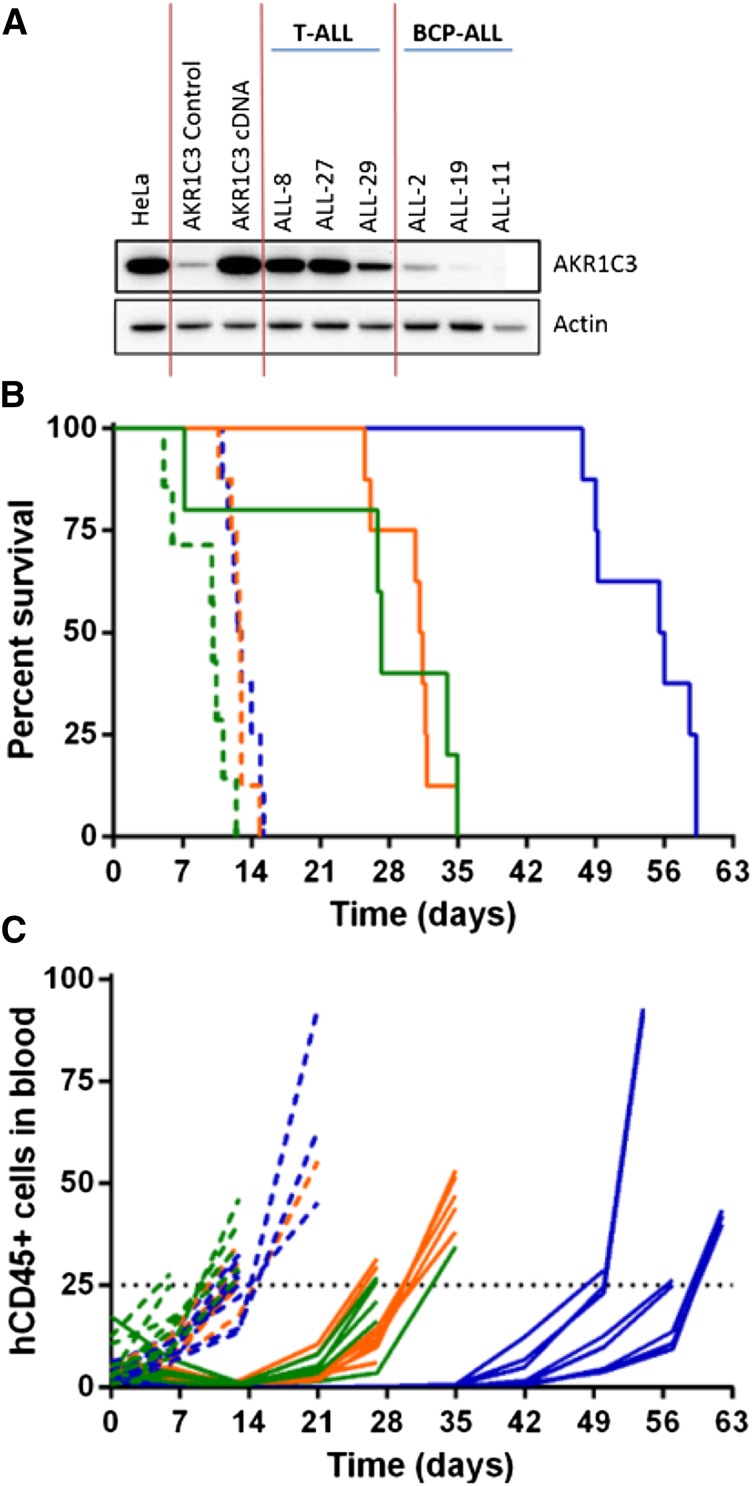
To demonstrate that AKR1C3 confers sensitivity to PR-104, a resistant BCP-ALL xenograft (ALL-11) was chosen for enforced gene expression experiments, as previously published.30 ALL-11 was transduced with the full-length AKR1C3 complementary DNA (cDNA) transcript resulting in expression of AKR1C3 protein to levels comparable with other T-ALL xenografts (Figure 4A). Overexpression of AKR1C3 cDNA did not alter in vitro sensitivity to dexamethasone, vincristine, daunorubicin, or l-asparaginase (supplemental Figure 5). PR-104 treatment of mice engrafted with ALL-11 untransduced and empty vector–transduced cells resulted in an LGD of 17.1 and 18.4 days, respectively, and both were scored with an ORM of a PR (Figure 4B-C; Table 3). In contrast, ALL-11 AKR1C3 cDNA-inoculated mice showed a CR with an LGD of 43.0 days. These results are consistent with published data showing that AKR1C3 overexpression resulted in a ninefold sensitization of ALL-11 cells to PR-104A in vitro.30 These data confirm that AKR1C3 overexpression significantly increased the in vivo sensitivity of ALL cells to PR-104 compared with the empty vector (P < .0001, log-ranked Mantel-Cox test for survival).
Figure 4.
In vivo response to PR-104 in an AKR1C3-overexpressing BCP-ALL xenograft. AKR1C3 cDNA was transduced into ALL-11 (BCP-ALL) resulting in higher levels of AKR1C3 protein expression, comparable to levels observed in T-ALL xenografts (A). These cells were then engrafted into NSG mice, and when the proportion of hCD45 cells in the PB reached 1%, mice were treated with PR-104 at 200 mg/kg for 2 weeks. (B) Kaplan-Meier plot shows the event-free survival of mice treated with PR-104 (solid lines) and engrafted with either the AKR1C3 cDNA (blue), the empty vector (orange), or the untransduced xenograft (green). PR-104 treatment significantly increased survival of AKR1C3 overexpressing mice compared with empty vector (Mantel-Cox analysis, P < .0001) Vehicle-treated mice appear in the broken lines. (C) Engraftment was monitored by the proportion of hCD45 cells in the PB of each mouse, and an event was recorded when it reached 25%.
Table 3.
Results of enforced expression of AKR1C3 in ALL-11 xenografted mice and treatment with PR-104 at 200 mg/kg for 2 weeks
| Treatment | EFS, d | LGD, d | P value PR-104 vs vehicle | Median ORM | Overall response | |
|---|---|---|---|---|---|---|
| Untransduced | Vehicle | 10.1 | ||||
| ALL-11 | PR-104 | 27.2 | 17.1 | .014 | 6 | PR |
| Empty vector | Vehicle | 12.9 | ||||
| control | PR-104 | 31.3 | 18.4 | <.0001 | 6 | PR |
| AKR1C3 | Vehicle | 12.8 | ||||
| cDNA | PR-104 | 55.8 | 43.0 | <.0001 | 9 | CR |
CR, complete response; EFS, event-free survival; LGD, leukemia growth delay; PR, partial response.
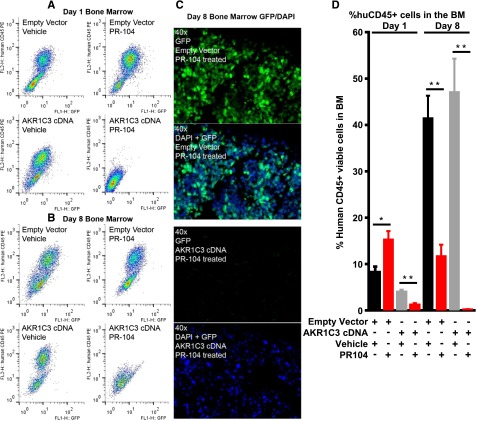
Further analysis was performed on the bone marrow infiltration of transduced cells, which express a GFP reporter. Mice inoculated with empty vector–expressing cells and treated with PR-104 showed a large population of hCD45+/GFP+ cells on flow cytometric analysis of the bone marrow 24 hours after the first dose (day 1, Figure 5A) or second dose (day 8, Figure 5B) of PR-104. In contrast, mice inoculated with the AKR1C3 cDNA-transduced ALL-11 cells showed almost complete disappearance of the hCD45+/GFP+ population at day 1 and day 8 (Figure 5A-B). Immunofluorescence analysis of day 8 bone marrow confirmed the almost complete absence of GFP+ cells, in contrast with the empty vector–transduced cells, which showed a high level of infiltration of GFP+ cells (Figure 5C). Quantitative data from n = 8 femurs are shown in Figure 5D. Overall, these data suggest that PR-104 can substantially reduce AKR1C3-expressing ALL cells from the bone marrow within 24 hours of treatment.
Figure 5.
Bone marrow infiltration of ALL-11 engrafted mice at day 1 and day 8. Bone marrow infiltration of mice engrafted with ALL-11 empty vector or AKR1C3 cDNA, both of which have GFP reporters. Samples were harvested 24 hours after the first dose of PR-104 (day 1, A) or 24 hours after the second dose of PR-104 (day 8, B) and cells were run on flow cytometry to determine the presence of GFP+ and hCD45+ cell populations. (C) Immunofluorescence for GFP in the bone marrow of PR-104–treated mice at day 8 shows absence of the GFP reporter in mice inoculated with cells transduced with AKR1C3 cDNA compared with empty vector. (D) Quantitative data showing the percentage of hCD45+ cells in n = 8 femurs. Data represent the mean ± SEM. *P < .01; **P ≤ .0005 by unpaired Student t test with Welch correction.
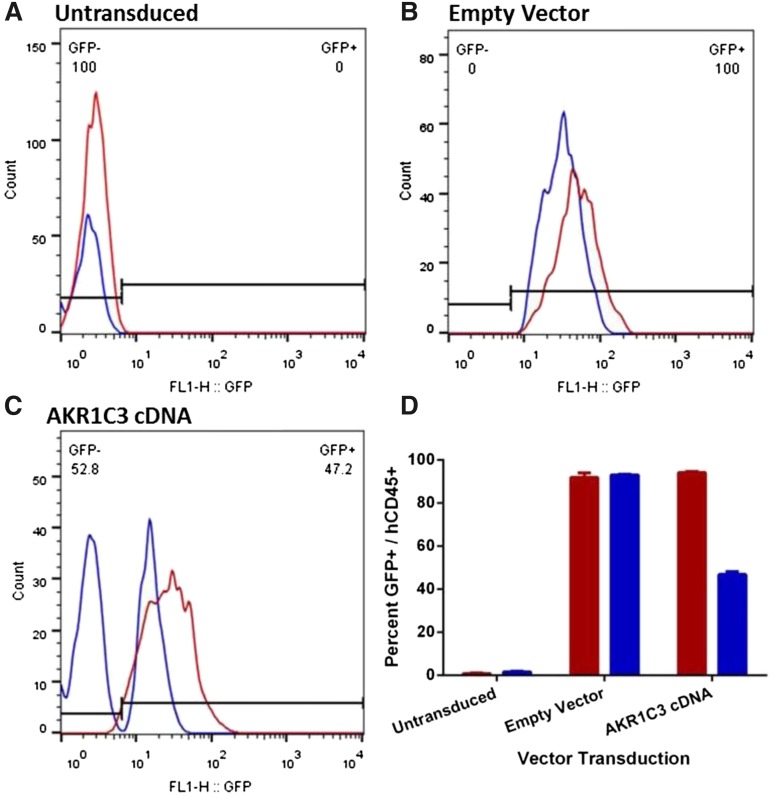
The peripheral blood (PB) of the mice was analyzed to determine the proportion of circulating GFP+ cells when the mice reached event (relapsed). As expected, GFP was not detected in mice engrafted with ALL-11–untransduced cells, whereas mice engrafted with ALL-11 cells expressing the empty vector had over 95% GFP+ cells in the PB in both the vehicle control or PR-104–treated groups (Figure 6A-B). Intriguingly, although vehicle-treated mice engrafted with ALL-11 cells expressing AKR1C3 cDNA maintained 95% of GFP+ cells in the PB when the mice reached event, treatment with PR-104 resulted in the emergence of a large GFP− population and only 46% GFP+ human CD45+ cells (Figure 6C-D). The dramatic decline of the GFP+ population in the AKR1C3 cDNA group following treatment with PR-104 suggests a strong negative selection for cells expressing AKR1C3 as a consequence of treatment. Therefore, because downregulation of AKR1C3 may be a mechanism of acquired resistance to PR-104, we next assessed AKR1C3 protein levels in 2 T-ALL xenograft cells (ALL-8 and ALL-31) harvested from the spleens of vehicle control and PR-104–treated mice at relapse. However, no differences were apparent in AKR1C3 expression between control and PR-104–treated samples (supplemental Figure 6).
Figure 6.
Proportion of human CD45+/GFP+ in the peripheral blood of ALL-11 engrafted mice at event. PB from mice engrafted with untransduced (A), empty vector (B), or AKR1C3 cDNA (C) ALL-11 cells was collected when mice reached event (hCD45+ population in blood reaches 25%). One representative mouse is shown from each. The hCD45+ population was gated by flow cytometry to determine the proportion of GFP+ cells circulating in the PR-104–treated (blue) or vehicle (red) groups. The numbers in panels A through C refer to the percentage of GFP− and GFP+ cells in the PR-104–treated mice. (D) Quantification of the proportion of hCD45+/GFP+ cells in all groups.
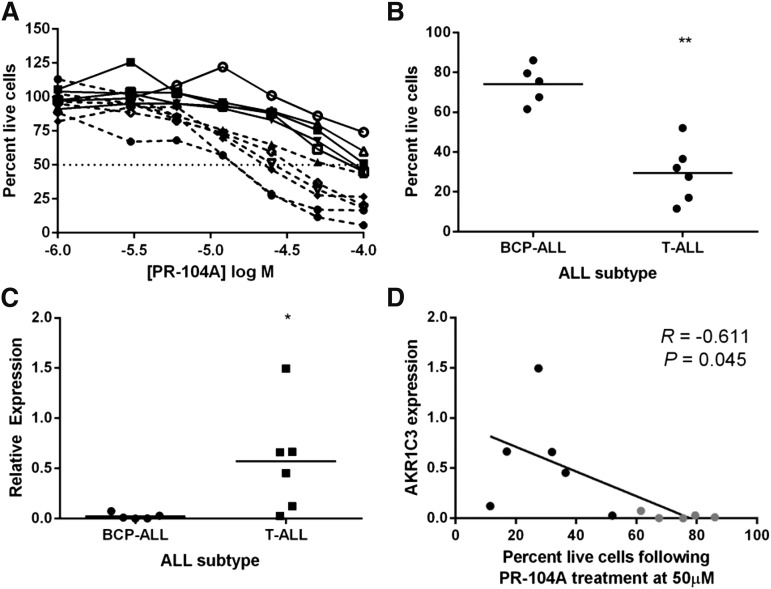
Primary T-ALL mononuclear cells are more sensitive to PR-104A than BCP-ALL
Mononuclear cells obtained at biopsy from 11 BCP-ALL or T-ALL patients were tested to validate the finding that AKR1C3 confers sensitivity to PR-104. Patient cells were tested in coculture with MSC-hTERT cells, and treated with PR-104A for 24 hours. Cell viability using 7AAD staining showed that BCP-ALL cells had a significantly higher proportion of live cells at 50 μM PR-104A compared with T-ALL cells (Figure 7A-B). It is unlikely that the sensitivity of BCP-ALL cells could be attributed to indirect effects because PR-104A at concentrations of up to 100 µM exerted no cytotoxicity against MSC-hTERT cells alone (supplemental Figure 7). AKR1C3 expression was measured by real-time quantitative reverse transcription–polymerase chain reaction (RT-qPCR), and expression was significantly greater in T-ALL compared with BCP-ALL patient cells (Figure 7C). Finally, we found a significant correlation between AKR1C3 mRNA expression and the proportion of live cells at 24 hours following 50 μM PR-104A treatment in coculture (Figure 7D; R = −0.61; P = .045). These data confirm that the differential expression of AKR1C3 influences PR-104 sensitivity of human ALL cells and that this phenomenon is not an artifact of xenografting.
Figure 7.
AKR1C3 expression correlates with PR-104A sensitivity in primary ALL cells. (A) Mononuclear cells purified from BCP-ALL (solid line) and T-ALL (broken line) patients were treated with PR-104A in coculture with MSC-hTERT cells in a dose response. Cells were stained with 7AAD 24 hours following treatment and percentages of live cells are shown relative to vehicle treatment. (B) Proportion of live cells after treatment with 50 μM PR-104A for 24 hours for BCP-ALL and T-ALL subtypes. (C) RT-qPCR expression of AKR1C3 in patient cells relative to ALL-8 xenograft control. (D) Correlation of AKR1C3 expression and sensitivity to 50 μM PR-104A in BCP-ALL (gray) and T-ALL (black) patient samples. Results shown are the mean of 2 biological repeats. *P < .05; **P ≤ .005 by Mann-Whitney U test.
Discussion
PR-104 represents a novel bifunctional DNA alkylating agent which exhibited greater potency against ALL compared with solid tumor models when tested by the Pediatric Preclinical Testing Program.33 Further testing suggested preferential efficacy against 1 T-ALL in comparison with 1 BCP-ALL xenograft.32 In this study, we used a combination of in vitro and in vivo models to show that PR-104 exhibited subtype specificity for T-ALL compared with BCP-ALL in a larger panel of patient-derived xenografts, and that expression of AKR1C3 was a strong predictive biomarker for sensitivity. A notable exception was the T-ALL xenograft ALL-42, which expressed low AKR1C3 levels and a sensitivity to PR-104A that was more comparable to BCP-ALL xenografts. The underlying mechanism for reduced AKR1C3 expression in ALL-42 is not currently understood, but this outlier indicates that AKR1C3 expression, rather than ALL subtype, is a more important indicator of PR-104 sensitivity. T-ALL mononuclear cells from 11 patients also showed significantly greater sensitivity to PR-104 compared with BCP-ALL, and this correlated with expression of AKR1C3. These data support a trial of PR-104 in a clinical setting for T-ALL patients with high AKR1C3 expression in their leukemic blasts.
PR-104 is a phosphate ester that is hydrolyzed systemically to release PR-104A, which was initially designed as a HAP that is reduced to its active metabolites in the absence of oxygen.18,20 The identification of AKR1C3 as an activator of PR-104A in aerobic as well as hypoxic cells provided a new potential biomarker for tumors sensitive to PR-104.21 Although there is already evidence for PR-104 efficacy in hypoxia in bone marrow infiltrated with ALL cells in patients and the xenograft model,32 validation of AKR1C3 as a biomarker for PR-104 sensitivity in aerobic conditions was required. In this study, we screened a panel of BCP-ALL and T-ALL patient-derived xenografts and found that AKR1C3 was a strong predictor of response to PR-104, independent of hypoxia. Overexpression of AKR1C3 in a BCP-ALL xenograft resulted in sensitivity to PR-104 in vivo, and PR-104 was effective at clearing the bone marrow of AKR1C3-overexpressing blasts within 24 hours of the first PR-104 dose, whereas PR-104 failed to clear the BCP-ALL blasts with the empty vector control from the bone marrow. This confirmed that the mechanism of action of PR-104 is more closely related to the expression of AKR1C3 rather than hypoxia in this setting.
The presence of AKR1C3 expression in malignant cells has prompted numerous laboratories to synthesize AKR1C3-specific inhibitors which could be effective in solid tumors or leukemia.42-45 Although synthesis of targeted inhibitors is a common approach to control malignant cell growth, Khanim et al found that the use of a specific inhibitor to AKR1C3 did not prevent tumor growth in an AML cell line following a high-throughput screen for AKR1C3 inhibitors.45 The value of a preprodrug such as PR-104 is that it specifically targets cells expressing AKR1C3, without requiring the malignant cell to be reliant on this pathway for survival. T-ALL presents with both high expression of AKR1C3 and hypoxia, making PR-104 an attractive treatment strategy for patients with T-ALL.
To enhance the clinical utility of novel agents, it is important that they do not interfere with the standard induction treatments commonly used, such as VXL treatment in ALL. In this study, we observed that combining PR-104 treatment with VXL did not attenuate the in vivo responses to these drugs, nor did it result in therapeutic enhancement. In addition, stable AKR1C3 overexpression in ALL-11 xenograft cells did not alter their sensitivity to commonly used chemotherapeutic drugs when tested in vitro. Taken together, these data suggest that PR-104 would not adversely interact with common induction treatments. Moreover, the selection of ALL patients based on high AKR1C3 expression would not bias toward a patient population whose disease may be more sensitive to established drugs.
PR-104 has already been studied in adult solid tumors and hematologic malignancies in early-phase clinical trials. A phase 1 study in patients with advanced solid tumors revealed that PR-104 was well tolerated at 1.1 g/m2 administered as a 1-hour IV infusion every 3 weeks. The majority of adverse events were mild or moderate in severity (grade 1 or 2), with dose-related myelosuppression being the major toxicity.46 PR-104 was also studied in a phase 1/2 trial against adult patients with relapsed/refractory AML or ALL. Patients with AML after 1 or 2 prior therapies, or ALL after any number of prior therapies, received PR-104 (1.1-4 g/m2) as a 1-hour IV infusion over multiple cycles. The most frequent treatment-related adverse events were myelosuppression, febrile neutropenia, infection, and enterocolitis. In that study, no CRs were observed at doses below 3 g/m2. However, PR-104 showed promising activity with 6 of 17 AML patients treated with doses above 3 g/m2 showing clinical responses.19,30 Although biomarker data were limited in this study, AML blasts have been shown to have high levels of AKR1C3,28 so that AKRIC3 may be a valuable biomarker in all acute leukemias.
The animal model used in this study is an established leukemia model for preclinical testing of new chemotherapies.38,47 Although patient-derived xenografts are an excellent drug discovery tool, we validated these findings using primary patient cells cultured in vitro in a coculture assay. The panel of 11 ALL patient samples mirrored closely the observations from the xenografts that AKR1C3 correlates with PR-104A sensitivity, and that T-ALL cells are more sensitive to PR-104 compared with BCP-ALL. In summary, this study has provided evidence that AKR1C3 is a biomarker that could be used to identify leukemia patients who may most benefit from treatment with PR-104 in a prospective clinical trial.
Acknowledgments
The Children’s Cancer Institute is affiliated with the University of New South Wales Australia and The Sydney Children’s Hospitals Network.
This work was supported by grants from the National Institutes of Health National Cancer Institute (NOI-CM-42216 and NOI-CM-91001-03), and the National Health and Medical Research Council of Australia (NHMRC). R.B.L. is supported by a fellowship from the NHMRC.
Footnotes
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: H.C., D.M.M., J.E.-H., W.R.W., P.J.H., M.A.S., and R.B.L. designed the study; H.C., D.M.M., J.E.-H., K.E., A.H., C.B., R.T.K., J.R., C.E.T., and L.S.B. generated and analyzed the data; R.S. and G.M.M. selected suitable patients and provided patient data; and J.E.-H., D.M.M., R.S., G.M.M., M.A.S., and R.B.L. interpreted the data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Richard B. Lock, Children’s Cancer Institute, Lowy Cancer Research Centre, UNSW Australia, PO BOX 81, Randwick NSW 2031, Australia; e-mail: rlock@ccia.unsw.edu.au.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354(2):166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 3.Graux C. Biology of acute lymphoblastic leukemia (ALL): clinical and therapeutic relevance. Transfus Apheresis Sci. 2011;44(2):183–189. doi: 10.1016/j.transci.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Smith MA, Altekruse SF, Adamson PC, Reaman GH, Seibel NL. Declining childhood and adolescent cancer mortality. Cancer. 2014;120(16):2497–2506. doi: 10.1002/cncr.28748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg JM, Silverman LB, Levy DE, et al. Childhood T-cell acute lymphoblastic leukemia: the Dana-Farber Cancer Institute acute lymphoblastic leukemia consortium experience. J Clin Oncol. 2003;21(19):3616–3622. doi: 10.1200/JCO.2003.10.116. [DOI] [PubMed] [Google Scholar]
- 6.Van Vlierberghe P, Ferrando A. The molecular basis of T cell acute lymphoblastic leukemia. J Clin Invest. 2012;122(10):3398–3406. doi: 10.1172/JCI61269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci USA. 2007;104(13):5431–5436. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winkler IG, Barbier V, Wadley R, Zannettino AC, Williams S, Lévesque JP. Positioning of bone marrow hematopoietic and stromal cells relative to blood flow in vivo: serially reconstituting hematopoietic stem cells reside in distinct nonperfused niches. Blood. 2010;116(3):375–385. doi: 10.1182/blood-2009-07-233437. [DOI] [PubMed] [Google Scholar]
- 9.Chow DC, Wenning LA, Miller WM, Papoutsakis ET. Modeling pO(2) distributions in the bone marrow hematopoietic compartment. II. Modified Kroghian models. Biophys J. 2001;81(2):685–696. doi: 10.1016/S0006-3495(01)75733-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giuntoli S, Rovida E, Gozzini A, et al. Severe hypoxia defines heterogeneity and selects highly immature progenitors within clonal erythroleukemia cells. Stem Cells. 2007;25(5):1119–1125. doi: 10.1634/stemcells.2006-0637. [DOI] [PubMed] [Google Scholar]
- 12.Eliasson P, Jönsson JI. The hematopoietic stem cell niche: low in oxygen but a nice place to be. J Cell Physiol. 2010;222(1):17–22. doi: 10.1002/jcp.21908. [DOI] [PubMed] [Google Scholar]
- 13.Frolova O, Samudio I, Benito JM, et al. Regulation of HIF-1α signaling and chemoresistance in acute lymphocytic leukemia under hypoxic conditions of the bone marrow microenvironment. Cancer Biol Ther. 2012;13(10):858–870. doi: 10.4161/cbt.20838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsunaga T, Imataki O, Torii E, et al. Elevated HIF-1α expression of acute myelogenous leukemia stem cells in the endosteal hypoxic zone may be a cause of minimal residual disease in bone marrow after chemotherapy. Leuk Res. 2012;36(6):e122–e124. doi: 10.1016/j.leukres.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 15.Guise CP, Mowday AM, Ashoorzadeh A, et al. Bioreductive prodrugs as cancer therapeutics: targeting tumor hypoxia. Chin J Cancer. 2014;33(2):80–86. doi: 10.5732/cjc.012.10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiss GJ, Infante JR, Chiorean EG, et al. Phase 1 study of the safety, tolerability, and pharmacokinetics of TH-302, a hypoxia-activated prodrug, in patients with advanced solid malignancies. Clin Cancer Res. 2011;17(9):2997–3004. doi: 10.1158/1078-0432.CCR-10-3425. [DOI] [PubMed] [Google Scholar]
- 17.Ganjoo KN, Cranmer LD, Butrynski JE, et al. A phase I study of the safety and pharmacokinetics of the hypoxia-activated prodrug TH-302 in combination with doxorubicin in patients with advanced soft tissue sarcoma. Oncology. 2011;80(1-2):50–56. doi: 10.1159/000327739. [DOI] [PubMed] [Google Scholar]
- 18.Patterson AV, Ferry DM, Edmunds SJ, et al. Mechanism of action and preclinical antitumor activity of the novel hypoxia-activated DNA cross-linking agent PR-104. Clin Cancer Res. 2007;13(13):3922–3932. doi: 10.1158/1078-0432.CCR-07-0478. [DOI] [PubMed] [Google Scholar]
- 19.Konopleva M, Thall PF, Arana Yi C, et al. Phase I/II study of the hypoxia-activated prodrug PR104 in refractory/relapsed acute myeloid leukemia and acute lymphoblastic leukemia. Haematologica. 2015;100:927–934. doi: 10.3324/haematol.2014.118455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guise CP, Abbattista MR, Tipparaju SR, et al. Diflavin oxidoreductases activate the bioreductive prodrug PR-104A under hypoxia. Mol Pharmacol. 2012;81(1):31–40. doi: 10.1124/mol.111.073759. [DOI] [PubMed] [Google Scholar]
- 21.Guise CP, Abbattista MR, Singleton RS, et al. The bioreductive prodrug PR-104A is activated under aerobic conditions by human aldo-keto reductase 1C3. Cancer Res. 2010;70(4):1573–1584. doi: 10.1158/0008-5472.CAN-09-3237. [DOI] [PubMed] [Google Scholar]
- 22.Penning TM, Drury JE. Human aldo-keto reductases: function, gene regulation, and single nucleotide polymorphisms. Arch Biochem Biophys. 2007;464(2):241–250. doi: 10.1016/j.abb.2007.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Penning TM, Burczynski ME, Jez JM, et al. Human 3alpha-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo-keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem J. 2000;351(Pt 1):67–77. doi: 10.1042/0264-6021:3510067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen WD, Zhang Y. Regulation of aldo-keto reductases in human diseases. Front Pharmacol. 2012;3:35. doi: 10.3389/fphar.2012.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penning TM, Byrns MC. Steroid hormone transforming aldo-keto reductases and cancer. Ann N Y Acad Sci. 2009;1155:33–42. doi: 10.1111/j.1749-6632.2009.03700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fung KM, Samara EN, Wong C, et al. Increased expression of type 2 3alpha-hydroxysteroid dehydrogenase/type 5 17beta-hydroxysteroid dehydrogenase (AKR1C3) and its relationship with androgen receptor in prostate carcinoma. Endocr Relat Cancer. 2006;13(1):169–180. doi: 10.1677/erc.1.01048. [DOI] [PubMed] [Google Scholar]
- 27.Stanbrough M, Bubley GJ, Ross K, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66(5):2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 28.Birtwistle J, Hayden RE, Khanim FL, et al. The aldo-keto reductase AKR1C3 contributes to 7,12-dimethylbenz(a)anthracene-3,4-dihydrodiol mediated oxidative DNA damage in myeloid cells: implications for leukemogenesis. Mutat Res. 2009;662(1-2):67–74. doi: 10.1016/j.mrfmmm.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 29.Mahadevan D, DiMento J, Croce KD, et al. Transcriptosome and serum cytokine profiling of an atypical case of myelodysplastic syndrome with progression to acute myelogenous leukemia. Am J Hematol. 2006;81(10):779–786. doi: 10.1002/ajh.20690. [DOI] [PubMed] [Google Scholar]
- 30.Jamieson SM, Gu Y, Manesh DM, et al. A novel fluorometric assay for aldo-keto reductase 1C3 predicts metabolic activation of the nitrogen mustard prodrug PR-104A in human leukaemia cells. Biochem Pharmacol. 2014;88(1):36–45. doi: 10.1016/j.bcp.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 31.Desmond JC, Mountford JC, Drayson MT, et al. The aldo-keto reductase AKR1C3 is a novel suppressor of cell differentiation that provides a plausible target for the non-cyclooxygenase-dependent antineoplastic actions of nonsteroidal anti-inflammatory drugs. Cancer Res. 2003;63(2):505–512. [PubMed] [Google Scholar]
- 32.Benito J, Shi Y, Szymanska B, et al. Pronounced hypoxia in models of murine and human leukemia: high efficacy of hypoxia-activated prodrug PR-104. PLoS One. 2011;6(8):e23108. doi: 10.1371/journal.pone.0023108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Houghton PJ, Lock R, Carol H, et al. Initial testing of the hypoxia-activated prodrug PR-104 by the pediatric preclinical testing program. Pediatr Blood Cancer. 2011;57(3):443–453. doi: 10.1002/pbc.22921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel K, Choy SS, Hicks KO, Melink TJ, Holford NH, Wilson WR. A combined pharmacokinetic model for the hypoxia-targeted prodrug PR-104A in humans, dogs, rats and mice predicts species differences in clearance and toxicity. Cancer Chemother Pharmacol. 2011;67(5):1145–1155. doi: 10.1007/s00280-010-1412-z. [DOI] [PubMed] [Google Scholar]
- 35.Szymanska B, Wilczynska-Kalak U, Kang MH, et al. Pharmacokinetic modeling of an induction regimen for in vivo combined testing of novel drugs against pediatric acute lymphoblastic leukemia xenografts. PLoS One. 2012;7(3):e33894. doi: 10.1371/journal.pone.0033894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Houghton PJ, Morton CL, Gorlick R, et al. Stage 2 combination testing of rapamycin with cytotoxic agents by the Pediatric Preclinical Testing Program. Mol Cancer Ther. 2010;9(1):101–112. doi: 10.1158/1535-7163.MCT-09-0952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Houghton PJ, Morton CL, Tucker C, et al. The pediatric preclinical testing program: description of models and early testing results. Pediatr Blood Cancer. 2007;49(7):928–940. doi: 10.1002/pbc.21078. [DOI] [PubMed] [Google Scholar]
- 38.Liem NL, Papa RA, Milross CG, et al. Characterization of childhood acute lymphoblastic leukemia xenograft models for the preclinical evaluation of new therapies. Blood. 2004;103(10):3905–3914. doi: 10.1182/blood-2003-08-2911. [DOI] [PubMed] [Google Scholar]
- 39.Rose WC, Wild R. Therapeutic synergy of oral taxane BMS-275183 and cetuximab versus human tumor xenografts. Clin Cancer Res. 2004;10(21):7413–7417. doi: 10.1158/1078-0432.CCR-04-1045. [DOI] [PubMed] [Google Scholar]
- 40.Suryani S, Carol H, Chonghaile TN, et al. Cell and molecular determinants of in vivo efficacy of the BH3 mimetic ABT-263 against pediatric acute lymphoblastic leukemia xenografts. Clin Cancer Res. 2014;20(17):4520–4531. doi: 10.1158/1078-0432.CCR-14-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bachmann PS, Gorman R, Papa RA, et al. Divergent mechanisms of glucocorticoid resistance in experimental models of pediatric acute lymphoblastic leukemia. Cancer Res. 2007;67(9):4482–4490. doi: 10.1158/0008-5472.CAN-06-4244. [DOI] [PubMed] [Google Scholar]
- 42.Skarydova L, Hofman J, Chlebek J, et al. Isoquinoline alkaloids as a novel type of AKR1C3 inhibitors. J Steroid Biochem Mol Biol. 2014;143:250–258. doi: 10.1016/j.jsbmb.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Endo S, Hu D, Matsunaga T, et al. Synthesis of non-prenyl analogues of baccharin as selective and potent inhibitors for aldo-keto reductase 1C3. Bioorg Med Chem. 2014;22(19):5220–5233. doi: 10.1016/j.bmc.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 44.Flanagan JU, Atwell GJ, Heinrich DM, et al. Morpholylureas are a new class of potent and selective inhibitors of the type 5 17-β-hydroxysteroid dehydrogenase (AKR1C3). Bioorg Med Chem. 2014;22(3):967–977. doi: 10.1016/j.bmc.2013.12.050. [DOI] [PubMed] [Google Scholar]
- 45.Khanim F, Davies N, Veliça P, et al. Selective AKR1C3 inhibitors do not recapitulate the anti-leukaemic activities of the pan-AKR1C inhibitor medroxyprogesterone acetate. Br J Cancer. 2014;110(6):1506–1516. doi: 10.1038/bjc.2014.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jameson MB, Rischin D, Pegram M, et al. A phase I trial of PR-104, a nitrogen mustard prodrug activated by both hypoxia and aldo-keto reductase 1C3, in patients with solid tumors. Cancer Chemother Pharmacol. 2010;65(4):791–801. doi: 10.1007/s00280-009-1188-1. [DOI] [PubMed] [Google Scholar]
- 47.Lock RB, Liem N, Farnsworth ML, et al. The nonobese diabetic/severe combined immunodeficient (NOD/SCID) mouse model of childhood acute lymphoblastic leukemia reveals intrinsic differences in biologic characteristics at diagnosis and relapse. Blood. 2002;99(11):4100–4108. doi: 10.1182/blood.v99.11.4100. [DOI] [PubMed] [Google Scholar]