Fig. 3.
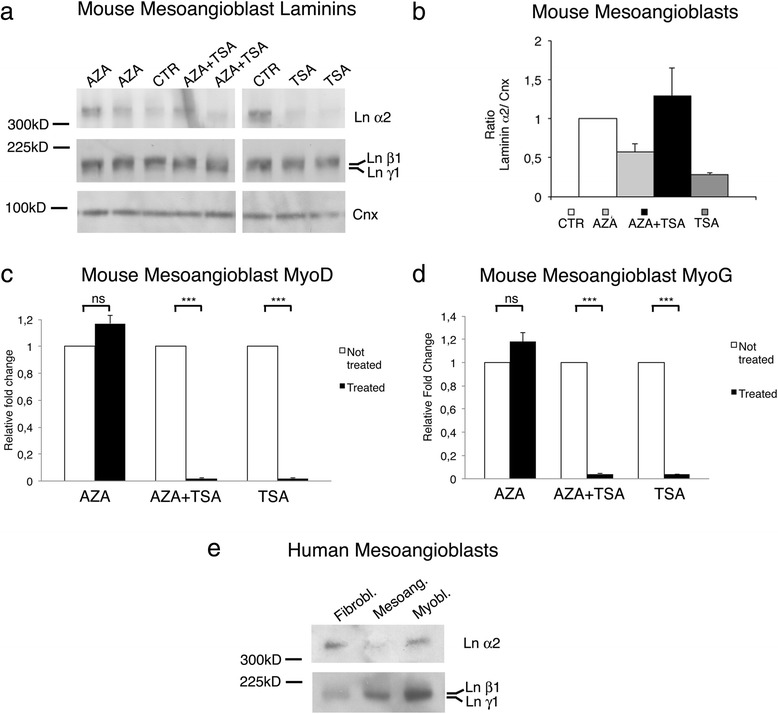
Human MABs and mouse MABs treated with deacetylating and demethylating substrates express very little amount of laminin α2 chain. a Western blot analysis of homogenates from differentiated mouse MABs (150 μg of protein loaded per lane) untreated (CTR) or treated with 5-aza-2′deoxycytidine (AZA), AZA and trichostatin A (AZA + TSA), or trichostatin A (TSA) for 3 days. Deacetylation (AZA) and/or demetylation (TSA) do not modify the expression of laminin chain a2, β1, and γ1. Calnexin (Cnx) is shown as reference for protein loading. b Quantification of densitometric results of the above the Western blot analysis as ratio between laminin chain α2 and calnexin, representing the mean of three determinations (±SEM). c–d Quantitative PCR analysis for MyoD (c) and MyoG (d) of differentiated MABs untreated or treated with AZA, AZA + TSA, or TSA; as expected MyoG was significantly increased by AZA and decreased by TSA treatment. e Western blot analysis of homogenates from human fibroblasts, human MABs, and human myoblasts. One hundred micrograms of protein were loaded per lane. Anti-laminin α2 chain antibody detects positive band in fibroblasts and myoblasts, whereas very little (if any) positive staining is present in human MABs. All three homogenates stained positive for laminin chain β1 and γ1
