Fig. 1.
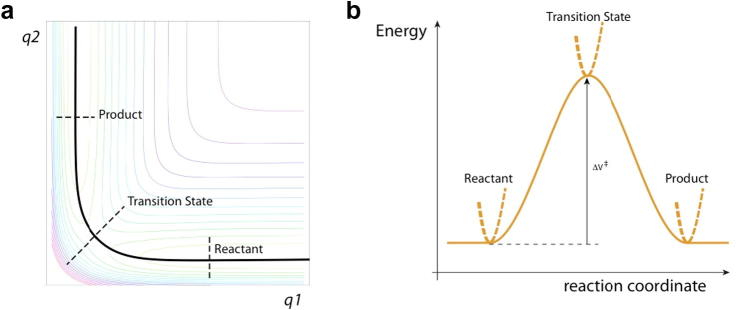
Schematic energy diagrams for a collinear, symmetric, gas phase A + BC → AB + C reaction, with A = C, and all masses equal for simplicity. (a) Contour potential energy diagram with q1 = the AB separation and q2 = the BC separation. Surfaces associated with the reactant (R), product (P) and symmetric transition state (TS, ‡) are shown. Solid line indicates the minimum energy path (MEP). (b) Plot of the energy along the MEP. The reaction coordinate x is the relative translation of the reactants in the R region, the ABC antisymmetric stretch at the TS, and the relative translation of the products in the P region. The dashed curves indicate the transverse, non-reactive coordinates: the BC stretch in the R region, the symmetric ABC stretch at the TS, and the AB stretch in the P region.
