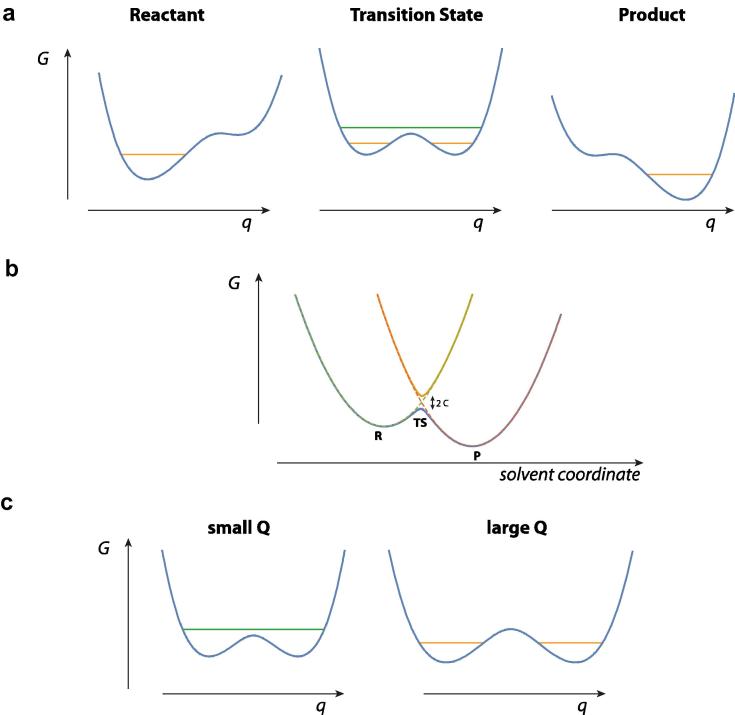
Fig. 8.
Schematic free energy (G) curves for the discussion of proton transfer reactions of type Eq. (4.1) in a polar environment, involving the proton coordinate q and the hydrogen-bond coordinate Q (a) proton potentials at different values of the environmental/solvent coordinate, with the proton vibrational levels shown (two different levels are shown for nonadiabatic tunneling and adiabatic cases at the transition state solvent coordinate value). (b) Free energy curves in the environmental/solvent coordinate; the reactant and product curves cross in the tunneling regime, but these are split by twice the proton coupling C to produce upper and lower curves in the adiabatic regimes. (c) Illustration of the aspect that the adiabatic regime is favored by small Q values (larger C), while the tunneling regime is favored by larger Q values (smaller C). The exponential behavior of C is especially important in the latter regime. See also e.g. [54,56].