Abstract
Nanoparticles (NP) are pervasive in many areas of modern life, with little known about their potential toxicities. One commercially important NP is cadmium oxide (CdO), which is used to synthesize other Cd-containing NP, such as quantum dots. Cadmium (Cd) is a well-known nephrotoxicant, but the nephrotoxic potential of CdO NP remains unknown, particularly when exposure occurs during pregnancy. Therefore, pregnant CD-1 mice were used to examine the effects of inhaled CdO NP (230 μg CdO NP/m3) on maternal and neonatal renal function by examining urinary creatinine and urinary biomarkers of kidney injury, including kidney injury molecule-1 (Kim-1) and neutrophil gelatinase-associated lipocalin (NGAL). Inhalation of CdO NP by dams produced a fivefold increase in urinary Kim-1 with no marked effect on urinary creatinine levels. Kim-1 mRNA expression peaked by gestational day (GD) 10.5, and NGAL expression increased from GD 10.5 to 17.5. In addition, histological analyses revealed proximal tubular pathology at GD 10.5. Neonatal Kim-1 mRNA expression rose between postnatal days (PND) 7 and 14, with mammary glands/milk being the apparent source of Cd for offspring. These studies demonstrate that, similar to what is seen with other Cd forms, Cd associated with inhaled CdO NP results in renal injury to both directly exposed dam and offspring. As commercial uses for nanotechnology continue to expand throughout the world, risks for unintentional exposure in the workplace increase. Given the large number of women in the industrial workforce, care needs to be taken to protect these already vulnerable populations.
Cadmium (Cd) compounds have long been used in the manufacture of batteries, dyes, and fire retardants, among other things. Recently, Cd compounds were also found to possess qualities that make them attractive in a wide range of industrial and medical uses as a nanoparticle (NP). Currently, nanosized Cd oxide (CdO) serves as the starting material in the manufacture of quantum dots, which are gaining favor in both medical diagnostic imaging and targeted therapeutics (Bentolila et al., 2005). Other uses for Cd-based nanomaterials include solar cells and biosensors (Halim, 2013; Malik et al., 2013). While the potential for occupational exposure to Cd-based NP remains a possibility, little is known about their toxicity or whether adverse effects (particularly regarding the kidney) are similar to those produced by other Cd forms. Importantly, as Cd is a well-known reproductive and developmental toxicant (Thompson and Bannigan, 2008), information concerning effects of inhaled Cd-containing NP on pregnant women and their offspring is critically needed to better protect these vulnerable populations, particularly in light of our previous studies demonstrating that Cd associated with inhaled CdO NP is translocated systemically from the point of deposition in the lungs to distant organs including the kidneys and placenta (Blum et al., 2012).
Cadmium compounds are considered to be potent environmental toxicants and classified by the International Agency for Research on Cancer (IARC) as human carcinogens (i.e., Group 1 carcinogen). Human exposure to Cd occurs primarily via dietary sources and inhalation of polluted environs, and is absorbed by the body in large quantities via cigarette smoke (Fels, 1999). Cd possesses a long biological half-life and accumulates particularly in the kidney, bone (Järup, 2002), and liver (Cupertino et al., 2013). In kidneys, chronic Cd exposure leads to nephrotoxicity characterized by proteinuria and polyuria (Ginsburg, 2012; Järup, 2002). While the proximal tubule is generally regarded as the primary site of Cd-induced renal injury, alterations in glomerular function also occur (Järup et al., 1995). Studies by Prozialeck et al. (2009) showed that Cd-induced proximal tubule injury is associated with elevated release of kidney injury molecule-1 (Kim-1). Release of Kim-1 into the urine was shown to increase before other changes in kidney function, such as decreased glomerular filtration rate and proteinuria. Kim-1, used previously for assessing gentamicin-(McWilliam et al., 2012), cisplatin- (McDuffie et al., 2013), and Cd-induced nephrotoxicity (Lee et al., 2014; Prozialeck et al., 2007, Pennemans et al., 2011), is a transmembrane protein not normally detected in kidney tissue, but becomes highly expressed following toxic insult (Prozialeck et al., 2009). After injury, Kim-1 is expressed by dedifferentiated cells in those areas in order to reform the epithelial layer in denuded areas of basement membrane. Associated with this process, the extracellular domain of Kim-1 is cleaved and excreted into the urine, where it may then be detected (Vaidya et al., 2008).
A second emerging marker used increasingly to assess acute renal injury is the presence of neutrophil gelatinase-associated lipocalin (NGAL, also known as lipocalin-2). Levels of NGAL expression in the kidney are normally low, but increase markedly as a result of renal cell damage (Supavekin et al., 2003). Elevated early in the etiology of acute kidney injury, resulting from ischemia (Mishra et al., 2003) or cisplatin exposure (Lin et al., 2013), NGAL was found to rise earlier than other, more commonly used urinary kidney injury markers such as creatinine, albumin, or cystatin C (Lin et al., 2013).
Exposure to stressors during development has been shown to result in physiologic changes to the developing offspring, changes that promote in utero survival (Thrifty Hypothesis of Barker: Barker and Martyn, 1992; Hales and Barker, 1992). While such changes enhance survival in utero, they may result in greater disease risk later in life. However, few studies have examined such outcomes following exposure to NP. An investigation that examined the effects of titanium dioxide exposure during pregnancy in rats revealed microvascular impairments in the maternal uterine arterioles that resulted in cardiac mitochondrial insufficiency in the offspring that persisted through adulthood (Stapleton et al., 2013, 2014). In our previous studies, pregnant mice were exposed by inhalation to CdO NP, and while Cd was not measurable in the fetus, there were effects on neonatal growth (Blum et al., 2012) and sex-dependent dyslipidemia in adult offspring fed a high-fat diet (report in preparation). Changes arising from stressors from development have also been associated with adult hypertension and kidney disease in humans (Zandi-Nejad et al., 2006). The primary goal of this offshoot study was to test the hypothesis that short-term, repeated inhalation exposure of pregnant mice to CdO NP (from gestational day [GD] 4.5 through GD 16.5) results in kidney injury in the mother and neonate, as evidenced by changes in urinary levels and/or mRNA expression of Kim-1 and NGAL in neonatal kidneys.
MATERIALS AND METHODS
Generation and Characterization of CdO NP
Cadmium oxide NP were generated as previously described (Blum et al., 2012, 2014) using a Palas arc generation system. The average NP concentration in this study was approximately 8.6 × 106 NP/cm3 with an NP surface area concentration of approximately 9.7 × 109 nm2/cm3 and a mean diameter of 15.3 nm (±1.6 nm geometric standard deviation indicative of polydispersed NP); metal content was >99% Cd as measured by x-ray fluorescence spectroscopy (XRF) (Blum et al., 2012). The efficiency of particle removal was monitored via XRF analysis of Teflon collection filters and real-time monitoring of the filtered carrier air using a scanning mobility particle sizer (SMPS) consisting of an electrostatic classifier (model 3080), a nano-differential mobility analyzer (DMA; model 3085, which has a detection range of 2–150 nm), and a condensation particle counter (model 3010) (TSI, St. Paul, MN). A typical CdO NP aerosol distribution curve is shown in Figure 1. Using a model 3081 DMA (detection range 10–1000 nm) did not reveal larger sized agglomeration of the particles (data not shown).
FIGURE 1.
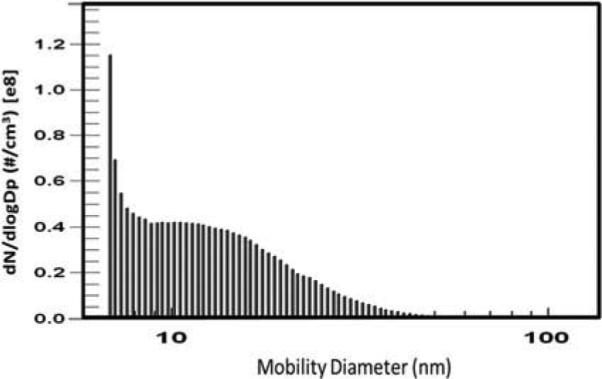
Representative particle size distribution of CdO NP generated for exposure of pregnant mice. The mean particle diameter was 15.3 ± 1.6 nm using a model 3085 DMA as described in the Materials and Methods section. This profile was maintained for each of the daily 2.5-h exposure runs.
Animals and CdO NP Exposure
Kidneys and urine collected from CdO NP-exposed dams and their offspring were collected from a previous study (Blum et al., 2012) and frozen at −80°C or −20°C, respectively, and used for these studies. In that study, mated CD-1 mice (Charles River Laboratories, Kingston, NY; 9–10 wk of age and approximately 26 g at the start of the exposure) were exposed by (nose-only) inhalation to ~230 μg CdO NP/m3 for 2.5 h/d, 7 d/wk, beginning on GD 4.5 (observation of seminal plug = GD 0.5) through either GD 9.5, 13.5, or 16.5. Control mice were exposed to cooled carrier air from which the NP were removed via high-efficiency particulate air filters prior to entering the control chamber (Blum et al., 2012).
At the appropriate postexposure time point, dams were either euthanized via intraperitoneal injection of sodium pentobarbital (120 mg/kg) 24 h after the final exposure or allowed to give birth (only those mice whose exposure continued through GD 16.5). Following euthanasia, kidneys were weighed and half of one maternal kidney was placed in formalin, while the other half was snap frozen in liquid nitrogen and stored at −80°C until used for analysis. A subset of dams, exposed to CdO NP from GD 4.5 through GD 16.5, was allowed to give birth. Randomly selected neonates were euthanized (as for the dams) on postnatal days (PND) 7, 10, or 14, and urine and kidneys were collected and stored at −20°C or −80°C, respectively. At the time of euthanasia, urine was collected directly from the urinary bladder using a 1-ml syringe. An experimental design and timeline is shown in Figure 2. All aspects of the care and use of experimental animals were reviewed and approved by the New York University Institutional Animal Care and Use Committee.
FIGURE 2.
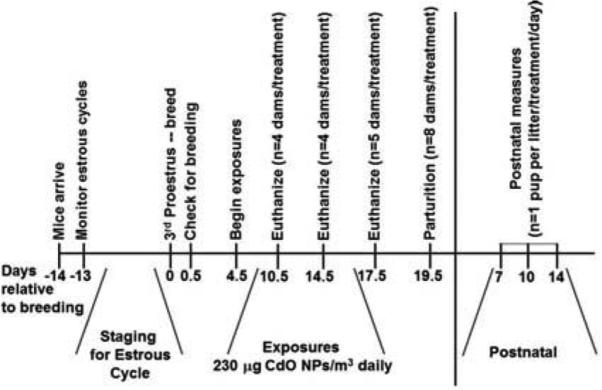
Experimental timeline for generating pregnant CD-1 mice and subsequent exposures to CdO NP by inhalation. Beginning on GD 4.5, mice were weighed, placed into nose-only exposure tubes, and placed on the exposure apparatus for 2.5 h/d, 7 d/wk. Some mice were exposed through GD 9.5, 13.5, or 16.5 and euthanized 1 d later, while the remaining mice gave birth on GD 19.5. For these studies, some neonates were euthanized for to assess their kidneys on postnatal days 7, 10, and 14.
Cadmium Levels in Maternal Mammary Glands
Cervical and thoracic mammary glands were collected from pregnant mice euthanized at GD 17.5, snap frozen in liquid nitrogen, and stored at −80°C until used to measure tissue Cd levels. To determine Cd burden, mammary-gland tissue was weighed, digested in hot (100–110°C) nitric acid, neutralized with hydrogen peroxide, and reduced to a total volume to 0.5 ml by boiling, and the final solution was reconstituted to 2 ml with Milli-Q water. Total Cd levels were determined by graphite furnace atomic absorption spectroscopy (gfAAS) as previously described (Blum et al., 2012); the minimum detection limit for gfAAS was 0.02 ng Cd/ml. Mammary gland Cd levels are presented as nanograms Cd per milligram wet tissue weight.
Measurement of Urinary Kim-1, Creatinine, and Glucose
Urine samples (stored at −20°C for ~6 mo) from dams (collected at GD 17.5) and neonates were analyzed for Kim-1 using a commercially available enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN) and as directed by the manufacturer. Urinary creatinine concentration was determined by the Jaffe reaction using a colorimetric assay against a standard curve using creatinine (Prozialeck et al., 2009; Shoucri and Pouliot, 1977). Glucose in the urine was measured using a glucose assay kit (Abcam, Cambridge, MA).
mRNA Expression of Kim-1 and NGAL in the Kidney
Total kidney RNA from dams and offspring (stored at −80°C for approximately 10 mo prior to analysis) was extracted using Trizol (Life Technologies) according to the manufacturer's directions. Following extraction, RNA samples were assessed for quantity and purity using a Nanodrop 2000 Spectrophotometer (Thermo Scientific). All RNA samples were DNase-treated (Turbo DNA-free, Ambion) to remove any trace DNA from the sample, and then reassessed for concentration and purity (all samples had 260/280-nm ratios between 1.8 and 2). Complementary DNAs (cDNA) were synthesized using the Improm-II reverse transcription system (Promega) according to the manufacturer's instructions. Reverse transcription reactions contained (per 25-μl reaction) 1 μg RNA, 5 mM MgCl2, 1 μl RNasin (Promega), 0.08 mM dNTP, and 120 ng random hexamer primers (Thermo Scientific). Gene expression levels in the cDNA samples were measured by real-time polymerase chain reaction (PCR; using an Applied Biosystems 7900 HT Fast Realtime PCR system). Samples were assayed in 10-μl reactions containing 1 μl cDNA and 0.25 μM primers using Power Sybr Green Master Mix (Applied Biosystems). Primer sequences are listed in Table 1.
TABLE 1.
Real-Time PCR Primers
| 5′ → 3′ | Annealing temperature (° C) | Reference | ||
|---|---|---|---|---|
| 18S | Forward | CGGCTACCACATCCAAGGAA | 61 | Blum et al. (2008) |
| Reverse | CCTGTATTGTTATTTTTCGTCACTACCT | |||
| Kim-1 | Forward | GGAAGTAAAGGGGGTAGTGGG | 61 | Krishnamoorthy et al. (2010) |
| Reverse | AAGCAGAAGATGGGCATTGC | |||
| NGAL | Forward | GCAGGTGGTACGTTGTGGG | 61 | PrimerBankIDa : 34328048c1 |
| Reverse | CTCTTGTAGCTCATAGATGGTGC |
Reference for PrimerBank is Spandidos et al. (2010).
Kidney Histopathology
Maternal kidneys recovered from dams at GD 10.5 (n = 3–4/treatment group) were fixed in formalin and shipped to Colorado Histo-Prep (Fort Collins, CO) for embedding, sectioning (5 μm), and staining with hematoxylin and eosin (H&E). Tissue sections were viewed by light microscopy using a Nikon E400 epifluorescent microscope. Images were captured with a 40× objective using an Evolution MP digital air-cooled color camera equipped with Image Pro Plus (v. 7.0) image acquisition software (MediaCybernetics, Rockville, MD). The scale bar indicates a length of 100 μm. To evaluate consistency of these morphologic changes from one tissue sample to another, three images were captured from random fields of the renal cortex from each sample.
Statistical Analysis
Statistical analyses were performed using SAS (v 9.1.3, SAS Institute, Cary, NC). All data were analyzed by one-way analysis of variance (ANOVA) with treatment and time (where appropriate) as main effects. Real-time PCR values were compared for each treatment group by calculating the ΔCt (change in threshold cycle) values for each individual sample and subtracting the Ct value for 18S rRNA from the Ct values for both Kim-1 and NGAL. Real-time PCR expression data, presented as ΔCt values, were used for comparison between treatment groups using one-way ANOVA. A lower ΔCt value represents a higher level of expression. Fold changes presented in the text were computed using the formula 2−ΔΔCt. When appropriate (ANOVA p value < .05), post hoc testing was performed using Fisher's least significant difference (LSD) test. Data presented are means ± SE.
RESULTS
Kim-1 Levels in Maternal Urine
The levels of urinary Kim-1 following inhalation exposure of pregnant mice to CdO NP from GD 4.5 through GD 16.5 increased by 4.7-fold compared to filtered-air exposed control mice (Figure 3A). The rise occurred in the absence of any significant change in urinary creatinine concentrations (Figure 3B). In addition, there was no significant effect on urinary glucose levels between control and NP-exposed mice.
FIGURE 3.
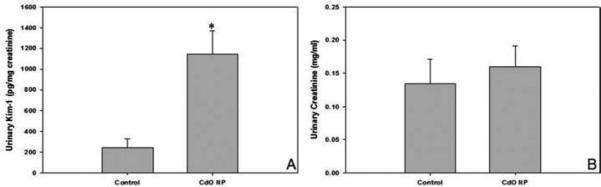
Inhalation of CdO NP (230 μg/m3) increased urinary excretion of Kim-1 in the dam. Pregnant mice were euthanized on GD 17.5 following daily exposures to CdO NP from GD 4.5 to 16.5. Urine was collected by direct aspiration of the urinary bladder. Urinary Kim-1 (A) was assayed using an ELISA, and creatinine levels (B) were measured using the Jaffee reaction as described in Materials and Methods section. Data are means ± SE of n = 3–4 individual mice per treatment. Significance: *p < .05.
Maternal Kidney mRNA Expression of Kim-1 and NGAL
Changes in kidney mRNA expression of Kim-1 and NGAL were measured over time during pregnancy (Figure 4). Data shown reflect ΔCt values and thus lower values correspond to greater mRNA expression. Expression levels of Kim-1 in CdO NP-exposed pregnant females were significantly elevated by GD 10.5 compared to time-matched control (5.5 Ct units or 45.3-fold) (Figure 4A). Kim-1 mRNA expression remained constant over time despite continued maternal exposure to CdO NP (i.e., GD 14.5 and 17.5), suggesting a maximal response by GD 10.5 (Figure 4A). The lack of a time-dependent response in the CdO NP treatment group was in sharp contrast to the time-related increase in Kim-1 mRNA expression observed in control mice; in this case, Kim-1 mRNA expression rose by 4-fold (2 Ct units) between GD 10.5 and 14.5 and by 5.7-fold (2.5 Ct units) between GD 14.5 and 17.5.
FIGURE 4.
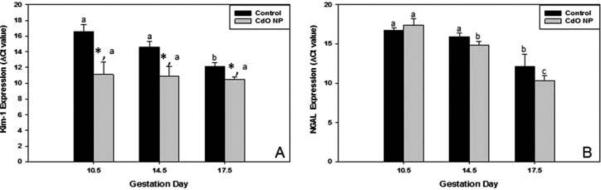
Exposure of mice to inhaled CdO NP (230 μg/m3) results in increased renal Kim-1 and NGAL mRNA expression during pregnancy. At designated times during exposure, pregnant mice were euthanized and the kidneys were collected. The mRNA expression levels were measured using real-time PCR for Kim-1 (A) and NGAL (B). Data are mean ΔCt values (normalized to 18S rRNA) ± SE from n = 3–5 individual mice per treatment per determination (lower ΔCt values represent higher levels of expression). Significance: *p < .05 between exposure groups on a particular gestation day; bars with different letters within a treatment group (i.e., control or CdO NP) are significantly different.
No significant changes in maternal expression of NGAL were observed (compared to control) as a result of CdO NP exposure (Figure 4B). However, for both sets of mice (i.e., NP-exposed and control), temporal changes in expression were observed within each given treatment group. The control group had a 9.2-fold increase in expression (3.2 Ct units) between GD 14.5 and 17.5, while no difference was observed between GD 10.5 and 14.5. In contrast, the temporal changes in the NP-exposed mice were more dramatic. In this case, there was a 6.5-fold increase (2.7 Ct units) between GD 10.5 and 14.5 and an 11.3-fold increase (3.5 Ct units) between GD 14.5 and 17.5 in NGAL expression.
Exposure to CdO NP Alters Proximal Tubule Morphology in Pregnant Mice
Representative images of H&E-stained maternal kidneys on GD 10.5 demonstrate that repeated inhalation exposure to CdO NP resulted in subtle morphological changes within the proximal tubules (Figure 5). Proximal tubules (arrows) from control kidney (Figure 5A) reveal dense cytoplasmic staining, with evenly spaced and well-defined nuclei; tubule lumens in control kidneys showed uniform cytoplasm with no apparent gaps or spaces between cells, which makes it difficult to discern borders between individual cells (solid arrows indicate individual round tubules). In contrast, proximal tubules from CdO NP-treated dams at the same gestational time point (Figure 5B) display less intense cytoplasmic (eosin) staining (i.e., broken arrows), small, irregularly shaped nuclei (denoted by broken ovals), and presence of gaps and/or vacuoles between tubular epithelial cells (indicated by asterisks).
FIGURE 5.
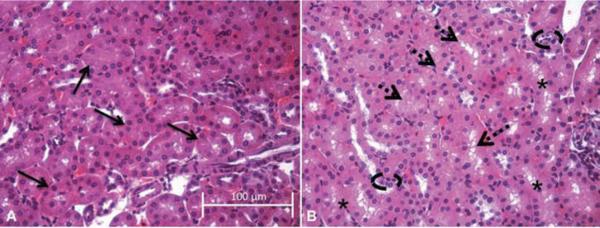
Representative hematoxylin and eosin (H&E) images showing changes in proximal tubule morphology. Images (40×) are representative of the results for n = 3 control aniumals (A) and n = 4 CdO NP-treated (B) animals at GD 10.5; scale bar indicates a length of 100 μm. Solid arrows in (A) designate individual round tubules in the control kidney section. Dashed arrows (B) denote tubules with less intense cytosolic staining, while broken ovals highlight areas of small, irregularly shaped nuclei; asterisks designate areas of gaps or vacuoles.
Mammary-Gland Cd Levels in Pregnant Mice
Mammary glands from mice exposed to inhaled CdO NP and recovered at GD 17.5 contained approximately fivefold higher Cd levels than that measured in control (Figure 6).
FIGURE 6.
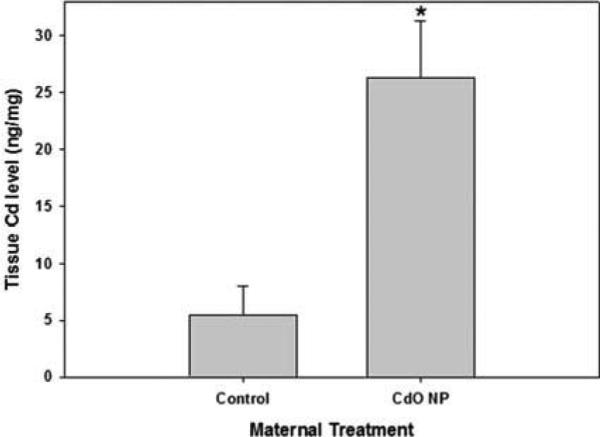
Inhalation of CdO NP resulted in Cd accumulation in the mammary glands of pregnant mice. Mice were exposed to CdO NP (230 μg/m3) from GD 4.5 to 16.5 and mammary tissue was collected on GD 17.5 for Cd determination. Data are means ± SE of n = 3–4 individual mice per treatment. Significance: *p < .05.
Kim-1 Levels in Neonatal Urine
Urine samples were collected from neonates and weanlings from PND 5 to 30. Due to small treatment sample sizes and limited sample volumes, data were pooled across ages. Concentrations of urinary Kim-1 in neonates born to NP-exposed mothers were increased by approximately 1.5-fold. However, due to extensive variability within treatment groups, likely due to relatively small sample sizes and mixed ages (n = 5 for control and n = 7 for NP-exposed), differences between the treatment groups failed to reach statistical significance (Figure 7A). Similar to that observed in dams, urinary creatinine levels in Cd-exposed offspring were not markedly different from control values (Figure 7B).
FIGURE 7.
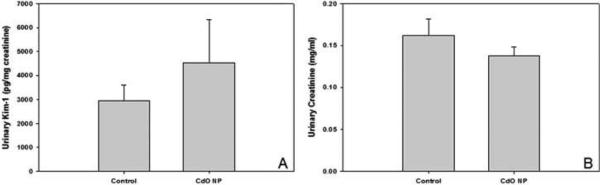
Kim-1 protein levels were increased in neonatal urine compared to that measured in control counterparts. Pregnant mice were exposed to CdO NP (230 μg/m3) from GD 4.5 to 16.5. Urine was collected from the urinary bladder of neonates euthanized between PND 5 and PND 31. Urine data were pooled from neonates of different ages to examine the effect of maternal exposure on urinary Kim-1 (A) and creatinine (B). Data are means ± SE for n = 5–7 individual pups from 3–4 different litters per treatment group.
Kidney mRNA Expression of Kim-1 and NGAL in Neonatal Offspring
By PND 10, neonatal offspring from mothers exposed to CdO NP had significantly higher Kim-1 mRNA expression than age-matched neonates born to control mothers (2.3-fold higher; 1.2 Ct units); mRNA expression remained significantly elevated in these same offspring (compared to controls) through PND 14 when the level of expression in exposed neonates was 3.2-fold (1.7 Ct units) higher (Figure 8A). In addition, Kim-1 expression in neonates from NP-exposed mothers increased significantly on PND 14 compared to PND 10 (2.8-fold; 1.5 Ct units), with similar changes not observed in their time-matched control counterparts. Expression of NGAL was unaffected by maternal exposure to CdO NP (compared to control) or as a result of PND differences within a treatment group (Figure 8B).
FIGURE 8.
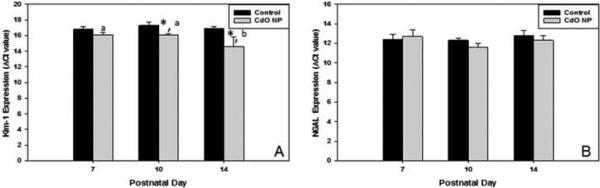
Response of Kim-1 and NGAL mRNA expression in neonates following maternal inhalation exposure to CdO NP during pregnancy. Neonatal kidneys were collected on PND 7, 10, and 14, and mRNA expression of Kim-1 (A) and NGAL (B) was assessed using real-time PCR. Data are mean ΔCt values normalized to 18S rRNA ± SE from n = 3–4 individual pups per determination (lower ΔCt values represent higher levels of expression) representing 3–4 different litters at each time point. Significance: *p < .05 between exposure groups on a particular gestation day; bars with different letters within a treatment group (i.e., control vs. CdO NP) are significantly different.
DISCUSSION
This study is an offshoot of a previous investigation that examined the pharmacokinetics and adverse health effects of inhaled CdO NP in mice during pregnancy (Blum et al., 2012). As women of reproductive age represent an increasing proportion of the workforce in the manufacturing sector, it is important to understand whether/how inhalation exposure to NP in general, and CdO NP specifically, impacts the health of pregnant women and their unborn children. Injury occurring early in life (either in utero and/or postpartum) is frequently irreversible (Makri et al., 2004; Ferguson et al., 2013), and therefore studies such as these are necessary to identify hazardous materials that can adversely influence sensitive subpopulations already at enhanced risk for toxic insult.
Exposure times during pregnancy and gestational days at which we examined kidney injury markers in the dam (GD 10.5, 14.5, and 17.5) were selected to most closely align with specific human pregnancy milestones. As previous studies (Yu and Chan, 1987) showed that exposure of in vitro fertilized embryos to soluble Cd diminished implantation, exposures began at GD 4.5 to allow for blastocyst transport and implantation into the uterus; GD 4.5 in mice is reflective of GD 5-9 in humans. By GD 10.5 (equivalent to approximately 1 mo gestation in humans), the placenta in mice has gained activity for both nutrient/waste transport and endocrine function for maintenance of pregnancy. In the mouse, GD 14.5 is the time when offspring have transitioned from the embryonic stage to a fetus and when only growth occurs for the remainder of pregnancy (reflective of approximately 10–12 wk in humans). GD 17.5 is a point near term when nearly all organs have matured and the majority of fetal growth has been completed (mid-third trimester in humans). These time points in mice represent periods during human pregnancy when offspring development/growth is at risk. The choice to examine the effects of pregnancy exposure of CdO NP on expression of kidney injury markers in the neonates was based on changes that occur in kidney function postnatally. Studies by Wu et al. (2013) reported that by PND 7, the neonatal mouse kidney has fully transitioned away from pathways expressed during embryonic development, and this process continues through PND 10 and 14 when the kidneys possess adult functions associated with molecular transport and lipid/energy metabolism.
The level of Cd measured in the lungs of exposed dams upon sacrifice on GD 17.5 was 16.0 ± 1.4 ng Cd/mg tissue (Blum et al., 2012). Assuming an average mouse respiration rate of 220 breaths per minute and 180 μl air per breath (Braun et al., 2004), daily inhalational intake of CdO NP in this study was approximately 1.7 μg (Blum et al., 2014). While no human CdO NP exposure data are available, the Occupational Safety & Health Administration (OSHA, 2012) action level for an 8-h shift for airborne Cd is 2.5 μg Cd/m3. Assuming this OSHA action level and that an average human worker inhales 6.3–7 m3 of air per 8-h work day (U.S. Environmental Protection Agency [EPA], 2011), a worker could be exposed to approximately 16.6 μg Cd per 8-h shift. Based on studies by Haddam et al. (2011) and Orisakwe et al. (2007), men working in the Cd industry had serum Cd levels of between 0.8 and 9 μg Cd/dl, which are greater than the serum Cd levels measured in the pregnant dams on GD 17.5 (<0.15 ng Cd/μl; Blum et al., 2012).
Exposure to soluble and insoluble Cd compounds results in extensive kidney injury (Chan et al., 1993; Dorian and Klaassen, 1995; Ginsberg, 2012). The classical view for such injury is that Cd, in the form of conjugates with metallothionein, cysteine, or glutathione, is readily filtered by the glomerulus and then taken up by epithelial cells of the proximal tubules. Cadmium–metallothionein conjugates, which get degraded within the renal proximal tubule cells, allow Cd to be released within the kidney, resulting in oxidative stress and/or alterations in epithelial cell adhesion (Dorian and Klaassen, 1995). Studies examining whether Cd from inhaled CdO NP results in elevated levels of oxidative stress in the kidneys at the present exposure levels (2.3 ng Cd/mg kidney in NP-exposed dams) are ongoing in our lab. These oxidative changes, in turn, lead to cellular death and loss of renal function (i.e., polyuria and proteinuria), resulting ultimately in kidney failure.
The majority of studies examining effects of Cd on rodent kidneys have focused on soluble forms such as cadmium chloride (CdCl2) (Prozialeck et al., 2007, 2009) or on larger, non-nanosized insoluble forms including CdO (Johri et al., 2010) that are normally injected or delivered to the animals via drinking water. Usually these model systems require a period of Cd exposure of weeks to months before alterations in kidney function are observed (Prozialeck et al., 2009). Prozialeck et al. (2009) demonstrated that levels of urinary Kim-1 in rats administered CdCl2 by subcutaneous (sc) injection (0.6 mg Cd/kg; 5 d/wk) were significantly elevated after 6 wk of exposure. In contrast to that seen in rats exposed sc to a soluble Cd form, maternal inhalation exposure to CdO NP during only the 3-wk duration of pregnancy increased urinary Kim-1. Moreover, histological alterations (i.e., loss of cell-cell adhesion within the renal proximal tubule epithelium) seen in this study and associated with elevated Kim-1 levels were similar to those described in chronic exposure studies using soluble Cd (Prozialeck et al., 2003, 2009). A comparison of these two studies suggests, based on the shorter exposure duration and lower concentration of CdO NP used for our studies, that Cd associated with CdO NP (or the CdO NP themselves) may be more nephrotoxic than soluble forms previously examined. Another possible reason for the difference in relative potency of Cd between the studies is the route of exposure. Cadmium compounds are absorbed more efficiently via inhalation compared to ingestion. When eaten or imbibed, the majority of Cd is excreted in feces, while only a small proportion of inhaled Cd is excreted in urine with the remaining being stored in the kidney and liver (Agency for Toxic Substances and Disease Registry [ATSDR], 2008; Bull 2010; Comité Scientifique de Toxicologie, Ecotoxicologie et l'Environnement [CSTEE], 2004). To better assess the differences between inhaled and ingested Cd and soluble Cd and Cd NP in producing kidney injury, future studies need to include comparison to inhaled nebulized ionic Cd with that following exposure to CdO NP by oral gavage.
A key issue that has yet to be resolved focuses upon the form(s) of Cd associated with CdO NP that are delivered to the maternal and neonatal kidney. As described previously (Johri et al., 2010), systemic exposure to Cd salts delivers the metal to the kidney primarily in the form of conjugates with metallothionein or low-molecular-weight thiols (i.e., cysteine or glutathione), although ionic Cd is the species most likely responsible for actual cellular injury (Chan et al., 1993; Dorian and Klaassen, 1995). The valence state of Cd that leaves the lungs in this study and reaches the kidneys (via the circulation) has yet to be elucidated, as the usual methods used to determine these factors (e.g., Raman spectroscopy) have failed to provide reliable results for CdO NP. Thus, it remains to be determined whether it is the CdO NP itself or the released Cd ion that reaches the kidney that produces renal injury. In an attempt to answer this question, dissolution studies were performed with freshly generated CdO NP that were incubated either in water or in artificial lung fluid with only minimal quantities of Cd released after 5 h (unpublished observations by our collaborators Drs. Alison Elder and Bob Gelein at the University of Rochester), suggesting that renal injury occurs through the action of the intact NP.
Kim-1 is thought to act as a regulator of cellular adhesion and endocytosis in regenerating cells of the injured proximal tubule as they reform a functional epithelial barrier. This process is associated with proteolytic cleavage of the ectodomain of Kim-1, which is shed into the urine. The presence of the cleaved ectodomain was shown to be a sensitive biomarker of renal injury induced by a variety of nephrotoxic agents including Cd (Viadya et al., 2008), but may also be a marker for increased renal proximal tubular cell proliferation (such as that seen during pregnancy). Although somewhat surprising, the finding here that Kim-1 and NGAL mRNA levels rose normally over time as pregnancy progressed might be attributed to changes that occur normally during pregnancy (i.e., elevated blood volume that results in increased renal bipolar diameter) (Ogueh et al., 2011). While it is not clear whether glomerular filtration rate is associated with cellular proliferation within the proximal tubule, it is possible that the number of epithelial cells within the proximal tubule rises, leading to temporal increases in Kim-1 expression such as that seen following acute kidney injury (Vaidya et al., 2008).
Although Cd levels in our previous studies using the same pregnant mouse model and exposure paradigm were below detectable limits in the developing fetus, whole-body Cd levels in offspring measured between PND 1 and PND 10 had Cd burdens ranging between 0.08 and 0.5 ng Cd/mg body weight, depending on the PND of testing (Blum et al., 2012). Examination of Cd burdens in the mammary glands of these same pregnant females (per milligram of tissue) demonstrated significantly greater levels at GD 17.5 (26.5 ng Cd/mg tissue) than that previously measured in maternal lungs (16.0 ng Cd/mg tissue). This finding suggests that mammary glands are a site for preferential deposition of Cd during pregnancy, and thus any CdO NP-induced kidney injury in the neonate could be a result of lactational exposure. This notion is supported by studies from Jacquillet et al. (2007), who demonstrated that kidney Cd levels in the offspring of pregnant rats administered CdCl2 (0.5 mg/kg/d) by oral gavage increased from PND 2 through PND 60. The authors suggest that the rise in kidney Cd levels in the rat offspring was due to deposition in the liver during lactation and subsequent release into the systemic circulation. Lactational transfer of Cd to the neonate was also demonstrated by Grawé and Oskarsson (2000), who showed that administration of 109CdCl2 (iv using osmotic minipumps) to pregnant rats increased radioactive Cd in milk and whole neonates. These findings, along with that of the present study, offer a potential route of toxicant delivery to the neonate that is not generally considered when occupational safety standards are developed and/or implemented.
Given the potent nephrotoxicity of other Cd forms, and the fact that mammary glands appear to act as a “reservoir” for Cd during pregnancy, the potential for acute kidney injury in the neonates was examined. Studies demonstrated that at PND 10 and 14, neonates born to mothers exposed to CdO NP had increased kidney levels of Kim-1 mRNA expression, although urinary Kim-1 protein levels were not significantly elevated (compared to age-matched control counterparts). A possible explanation for this is that, as observed following kidney injury, Kim-1 may be involved with cellular proliferation that occurs in the proximal tubules during postnatal growth of the kidney that are not manifested as changes in urinary Kim-1.
CONCLUSIONS
While many questions regarding the fate of CdO NP and their effects on Kim-1 processing have yet to be answered, these studies reveal that inhaled CdO NP increased Kim-1 mRNA expression in kidneys of both directly exposed pregnant females and their newborn offspring. Compared to previously published results describing nephrotoxicity of soluble Cd compounds, results of the current study suggest that Cd associated with inhaled CdO NP produces kidney injury equal to, or possibly greater than, that produced by other Cd compounds. The potential risk that exposure of pregnant women to inhaled CdO NP may carry to the next generation demands further study. Further, additional investigations regarding the functional significance of these findings and their implications for workplace safety are needed. This is especially true when confounding health conditions, such as diabetes, that affect kidney function are considered, as well as increasing evidence demonstrating that women and children may be especially susceptible to renal toxicity associated with exposure to low levels of Cd (Akesson et al., 2005; Jacquillet et al., 2007; Järup, 2002; Navas-Acien et al., 2009; Nawrot et al., 2008; Satarug et al., 2003; Suwazono et al., 2010; Thomas et al., 2009). The studies here, along with our previous investigations, demonstrate that inhalation exposure to CdO NP poses a potential public health threat to pregnant women and their offspring, and suggest that policies are needed to better protect the health and safety of female workers.
ACKNOWLEDGMENTS
The authors acknowledge the assistance of Carol Hoffman-Budde, Lori Horton, and Lauren Rosenblum for assistance with the animal exposures. In addition, the authors acknowledge Drs. Terry Gordon and Lung-Chi Chen (NYU School of Medicine) for providing the particle generation system used for these studies and their technical guidance during the animal exposures. We also thank Drs. Alison Elder and Bob Gelein (University of Rochester) for performing the in vitro CdO NP dissolution studies.
FUNDING
Supported by grant numbers R01-ES017427, T32-ES007324, and NYU NIEHS Center grant ES000260 from the National Institutes of Environmental Health Sciences.
REFERENCES
- Agency for Toxic Substances and Disease Registry . Toxicological profile for cadmium. Draft for public comment. U.S. Department of Health and Human Services; Atlanta, GA: 2008. [Google Scholar]
- Akesson A, Lundh T, Vahter M, Bjellerup P, Lidfeldt J, Nerbrand C, Samsioe G, Strömberg U, Skerfving S. Tubular and glomerular kidney effects in Swedish women with low environmental cadmium exposure. Environ. Health Perspect. 2005;113:1627–1631. doi: 10.1289/ehp.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Martyn CN. The maternal and fetal origins of cardiovascular disease. J. Epidemiol. Commun. Health. 1992;46:8–11. doi: 10.1136/jech.46.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentolila LA, Michalet X, Pinaud FF, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Quantum dots for molecular imaging and cancer medicine. Discov. Med. 2005;5:213–218. [PMC free article] [PubMed] [Google Scholar]
- Blum JL, Nyagode BA, James MO, Denslow ND. Effects of the pesticide methoxychlor on gene expression in the liver and testes of the male largemouth bass (Micropterus salmoides). Aquat. Toxicol. 2008;86:459–469. doi: 10.1016/j.aquatox.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum JL, Xiong JQ, Hoffman C, Zelikoff JT. Cadmium associated with inhaled cadmium oxide nanoparticles impacts fetal and neonatal development and growth. Toxicol. Sci. 2012;126:478–488. doi: 10.1093/toxsci/kfs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum JL, Rosenblum LK, Grunig G, Beasley MB, Xiong JQ, Zelikoff JT. Short-term inhalation of cadmium oxide nanoparticles alters pulmonary dynamics associated with lung injury, inflammation, and repair in a mouse model. Inhal. Toxicol. 2014;26:48–58. doi: 10.3109/08958378.2013.851746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull S. Cadmium toxicological overview. Health Protection Agency. Version 3. 2010 https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/337542/hpa_cadmium_toxicological_overview_v3.pdf.
- Braun A, Ernst H, Homann HG, Rittinghausen S. Respiratory tract. In: Hedrich H, editor. The laboratory mouse. 2nd ed. Elsevier Academic Press; New York, NY: 2004. pp. 225–243. [Google Scholar]
- Chan HM, Zhu LF, Zhong R, Grant D, Goyer RA, Cherian MG. Nephrotoxicity in rats following liver transplantation from cadmium-exposed rats. Toxicol. Appl. Pharmacol. 1993;123:89–96. doi: 10.1006/taap.1993.1225. [DOI] [PubMed] [Google Scholar]
- Comité Scientifique de Toxicologie, Ecotoxicologie et l'Environnement . Opinion on the results of the risk assessment of: cadmium metal human health and cadmium oxide human health carried out in the framework of Council Regulation (EEC) 793/93 on the evaluation and control of the risks of existing substances. CSTEE; 2004. http://ec.europa.eu/health/archive/ph_risk/committees/sct/documents/out228_en.pdf. [Google Scholar]
- Cupertino MC, Costa KL, Santos DC, Novaes RD, Condessa SS, Neves AC, Oliveira JA, Matta SL. Long-lasting morphofunctional remodelling of liver parenchyma and stroma after a single exposure to low and moderate doses of cadmium in rats. Int. J. Exp. Pathol. 2013;94:343–345. doi: 10.1111/iep.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorian C, Klaassen CD. Protection by zinc-metallothionein (ZnMT) against cadmium-metallothionein-induced nephrotoxicity. Fundam. Appl. Toxicol. 1995;26:99–106. doi: 10.1006/faat.1995.1079. [DOI] [PubMed] [Google Scholar]
- Fels LM. Risk assessment of nephrotoxicity of cadmium. Renal Fail. 1999;21:275–281. doi: 10.3109/08860229909085089. [DOI] [PubMed] [Google Scholar]
- Ferguson KK, O'Neill MS, Meeker JD. Environmental contaminant exposures and preterm birth: A comprehensive review. J. Toxicol. Environ. Health B Crit. Rev. 2013;16:69–113. doi: 10.1080/10937404.2013.775048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg GL. Cadmium risk assessment in relation to background risk of chronic kidney disease. J. Toxicol. Environ. Health A. 2012;75:374–390. doi: 10.1080/15287394.2012.670895. [DOI] [PubMed] [Google Scholar]
- Grawé KP, Oskarsson A. Cadmium in milk and mammary gland in rats and mice. Arch. Toxicol. 2000;73:519–527. doi: 10.1007/s002040050003. [DOI] [PubMed] [Google Scholar]
- Haddam N, Samira S, Dumont X, Taleb A, Lison D, Haufroid V, Bernard A. Confounders in the assessment of the renal effects associated with low-level urinary cadmium: An analysis in industrial workers. Environ. Health. 2011;10:37–46. doi: 10.1186/1476-069X-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: The thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- Halim MA. Harnessing sun's energy with quantum dots based solar cells. Nanomaterials. 2013;3:22–47. doi: 10.3390/nano3010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquillet G, Barbier O, Rubera I, Tauc M, Borderie A, Namorado MC, Martin D, Sierra G, Reyes JL, Poujeol P, Cougnon M. Cadmium causes delayed effects on renal function in the off-spring of cadmium-contaminated pregnant female rats. Am. J. Physiol. Renal Physiol. 2007;293:F1450–F1460. doi: 10.1152/ajprenal.00223.2007. [DOI] [PubMed] [Google Scholar]
- Järup L, Persson B, Elinder CG. Decreased glomerular filtration rate in solderers exposed to cadmium. Occup. Environ. Med. 1995;52:818–822. doi: 10.1136/oem.52.12.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järup L. Cadmium overload and toxi-city. Nephrol. Dial. Transplant. 2002;17(suppl. 2):35–39. doi: 10.1093/ndt/17.suppl_2.35. [DOI] [PubMed] [Google Scholar]
- Johri N, Jacquillet G, Unwin R. Heavy metal poisoning: The effects of cadmium on the kidney. Biometals. 2010;23:783–792. doi: 10.1007/s10534-010-9328-y. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy A, Clement ME, O'Leary E, Bonventre JV, Vaidya VS. TIM2 gene deletion results in susceptibility to cisplatin-induced kidney toxicity. Toxicol. Sci. 2010;118:298–306. doi: 10.1093/toxsci/kfq240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Park EY, Kim S, Son JY, Kim TH, Kang WG, Jeong TC, Kim KB, Kwack SJ, Lee J, Kim S, Lee BM, Kim HS. Evaluation of cadmium-induced nephrotoxicity using uri-nary metabolomic profiles in sprague-dawley male rats. J. Toxicol. Environ. Health A. 2014;77:1384–1398. doi: 10.1080/15287394.2014.951755. [DOI] [PubMed] [Google Scholar]
- Lin HY, Lee SC, Lin SF, Hsiao HH, Liu YC, Yang WC, Hwang DY, Hung CC, Chen HC, Guh JY. Urinary neutrophil gelatinase-associated lipocalin levels predict cisplatin-induced acute kidney injury better than albuminuria or urinary cystatin C levels. Kaohsiung J. Med. Sci. 2013;29:304–311. doi: 10.1016/j.kjms.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Makri A, Goveia M, Balbus J, Parkin R. Children's susceptibility to chemicals: A review by developmental stage. J. Toxicol. Environ. Health B Crit. Rev. 2004;7:417–435. doi: 10.1080/10937400490512465. [DOI] [PubMed] [Google Scholar]
- Malik P, Katyal V, Malik V, Asatkar A, Inwati G, Mukherjee TK. Nanobiosensors: Concepts and variations. ISRN Nanomater. 2013;10:327–35. doi:10.1155/2013/327435. [Google Scholar]
- McDuffie JE, Ma JY, Sablad M, Sonee M, Varacallo L, Louden C, Guy A, Vegas J, Liu X, La D, Snook S. Time course of renal proximal tubule injury, reversal, and related biomarker changes in rats following cisplatin administration. Int. J. Toxicol. 2013;32:251–260. doi: 10.1177/1091581813493013. [DOI] [PubMed] [Google Scholar]
- McWilliam SJ, Antoine DJ, Sabbisetti V, Turner MA, Farragher T, Bonventre JV, Park BK, Smyth RL, Pirmohamed M. Mechanism-based urinary biomarkers to identify the potential for aminoglycoside-induced nephrotoxicity in premature neonates: A proof-of-concept study. PLoS ONE. 2012;7:e43809. doi: 10.1371/journal.pone.0043809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J. Am. Soc. Nephrol. 2003;14:2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- Navas-Acien A, Tellez-Plaza M, Guallar E, Muntner P, Silbergeld E, Jaar B, Weaver V. Blood cadmium and lead and chronic kidney disease in US adults: A joint analysis. Am. J. Epidemiol. 2009;170:1156–1164. doi: 10.1093/aje/kwp248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrot TS, Van Hecke E, Thijs L, Richart T, Kuznetsova T, Jin Y, Vangronsveld J, Roels HA, Staessen JA. Cadmium-related mortality and long-term secular trends in the cadmium body burden of an environmentally exposed population. Environ. Health Perspect. 2008;116:1620–1628. doi: 10.1289/ehp.11667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Occupational Safety & Health Administration [January 18, 2015];Chemical Sampling Information/ Cadmium. 2012 http://www.osha.gov/dts/chemicalsampling/data/CH_223897.html.
- Ogueh O, Clough A, Hancock M, Johnson MR. A longitudinal study of the control of renal and uterine hemo-dynamic changes of pregnancy. Hypertens. Pregnancy. 2011;30:243–259. doi: 10.3109/10641955.2010.484079. [DOI] [PubMed] [Google Scholar]
- Orisakwe OE, Nwachukwu E, Osadolor HB, Afonne OJ, Okocha CE. Liver and kidney function tests amongst paint factory workers in Nkpor, Nigeria. Toxicol. Ind. Health. 2007;23:161–165. doi: 10.1177/0748233707081908. [DOI] [PubMed] [Google Scholar]
- Pennemans V, De Winter LM, Munters E, Nawrot TS, Van Kerkhove E, Rigo JM, Reynders C, Dewitte H, Carleer R, Penders J, Swennen Q. The association between urinary kidney injury molecule 1 and urinary cadmium in elderly during long-term, low-dose cadmium exposure: a pilot study. Environ. Health. 2011;10:77. doi: 10.1186/1476-069X-10-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prozialeck WC, Lamar PC, Lynch SM. Cadmium alters the localization of N-cadherin, E-cadherin, and beta-catenin in the proximal tubule epithelium. Toxicol. Appl. Pharmacol. 2003;189:180–195. doi: 10.1016/s0041-008x(03)00130-3. [DOI] [PubMed] [Google Scholar]
- Prozialeck WC, Vaidya VS, Li J, Waalkes MP, Edwards JR, Lamar PC, Bernard AM, Dumont X, Bonventre JV. Kidney injury molecule-1 is an early biomarker of cadmium nephrotoxicity. Kidney Int. 2007;72:985–993. doi: 10.1038/sj.ki.5002467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prozialeck WC, Edwards JR, Vaidya VS, Bonventre JV. Preclinical evaluation of novel urinary biomarkers of cadmium nephrotoxicity. Toxicol. Appl. Pharmacol. 2009;238:301–305. doi: 10.1016/j.taap.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satarug S, Baker JR, Urbenjapol S, Haswell-Elkins M, Reilly PE, Williams DJ, Moore MR. A global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Toxicol. Lett. 2003;137:65–83. doi: 10.1016/s0378-4274(02)00381-8. [DOI] [PubMed] [Google Scholar]
- Shoucri RM, Pouliot M. Some observations on the kinetics of the Jaffe reaction for creatinine. Clin. Chem. 1977;23:1527–1530. [PubMed] [Google Scholar]
- Spandidos A, Wang X, Wang H, Seed B. PrimerBank: A resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res. 2010;38:D792–D799. doi: 10.1093/nar/gkp1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton PA, Minarchick VC, Yi J, Engels K, McBride CR, Nurkiewicz TR. Maternal engineered nanomaterial exposure and fetal microvascular function: Does the Barker hypothesis apply? Am. J. Obstet. Gynecol. 2013;209(3):227, e1–e11. doi: 10.1016/j.ajog.2013.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton PA, Nichols CE, Yi J, McBride CR, Minarchick VC, Shepherd DL, Hollander JM, Nurkiewicz TR. Microvascular and mitochondrial dysfunction in the female F1 generation after gestational TiO2 nanoparticle exposure. Nanotoxicology. 2014;5:1–11. doi: 10.3109/17435390.2014.984251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supavekin S, Zhang W, Kucherlapati R, Kaskel FJ, Moore LC, Devarajan P. Differential gene expression following early renal ischemia/reperfusion. Kidney Int. 2003;63:1714–1724. doi: 10.1046/j.1523-1755.2003.00928.x. [DOI] [PubMed] [Google Scholar]
- Suwazono Y, Sand S, Vahter M, Skerfving S, Lidfeldt J, Akesson A. Benchmark dose for cadmium-induced osteoporosis in women. Toxicol. Lett. 2010;197:123–127. doi: 10.1016/j.toxlet.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Thomas LD, Hodgson S, Nieuwenhuijsen M, Järup L. Early kidney damage in a population exposed to cadmium and other heavy metals. Environ. Health Perspect. 2009;117:181–184. doi: 10.1289/ehp.11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J, Bannigan J. Cadmium: Toxic effects on the reproductive system and the embryo. Reprod. Toxicol. 2008;25:304–315. doi: 10.1016/j.reprotox.2008.02.001. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency [February 25, 2015];Exposure factors handbook. 2011 http://www.epa.gov/ncea/efh/pdfs/efh-chapter06.pdf.
- Vaidya VS, Waikar SS, Ferguson MA, Collings FB, Sunderland K, Gioules C, Bradwin G, Matsouaka R, Betensky RA, Curhan GC, Bonventre JV. Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin. Transl. Sci. 2008;1:200–208. doi: 10.1111/j.1752-8062.2008.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Sahoo D, Brooks JD. Comprehensive gene expression changes associated with mouse postnatal kidney development. J. Urol. 2013;189:2385–2390. doi: 10.1016/j.juro.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Yu HS, Chan ST. Effects of cadmium on preimplantation and early postim-plantation mouse embryos in vitro with special reference to their trophoblastic invasiveness. Pharmacol. Toxicol. 1987;60:129–134. doi: 10.1111/j.1600-0773.1987.tb01511.x. [DOI] [PubMed] [Google Scholar]
- Zandi-Nejad K, Luyckx VA, Brenner BM. Adult hypertension and kidney disease: the role of fetal programming. Hypertension. 2006;47:502–508. doi: 10.1161/01.HYP.0000198544.09909.1a. [DOI] [PubMed] [Google Scholar]


