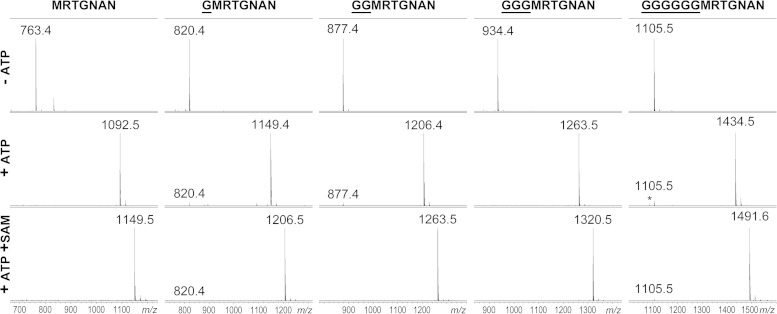
FIG 5.
In vitro adenylation of extended-length MccA peptide variants. Chemically synthesized peptides corresponding to wild-type MccA MRTGNAN and the indicated N-terminally extended variants were combined with recombinant MccB in the absence (top) and the presence (middle) of ATP. Reaction mixtures shown at the bottom were incubated in the presence of ATP, recombinant MccD, MccE, and Mtn enzymes, SAM substrate, and PLP cofactor under conditions promoting aminopropylation of peptidyl adenylates. The addition of aminopropyl adds 57 Da to adenylated peptides.