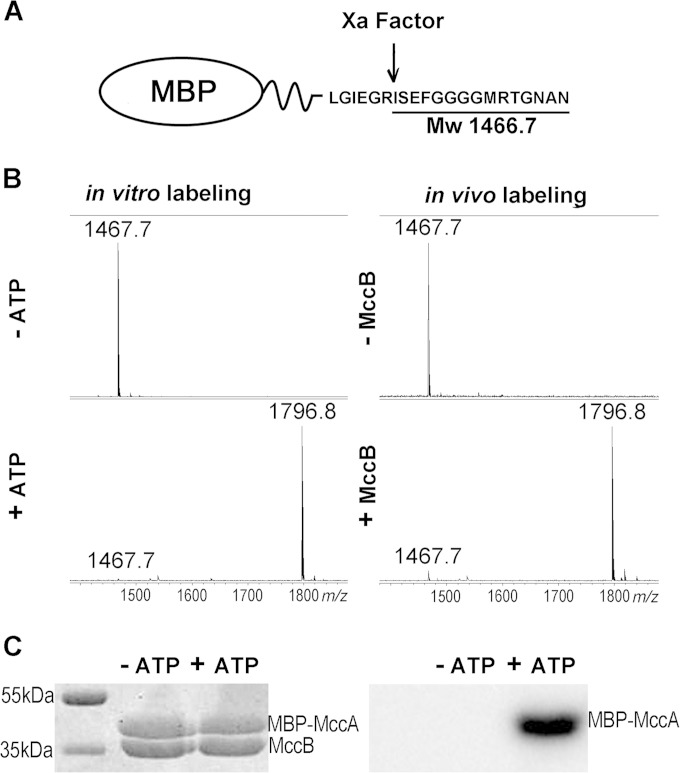
FIG 7.
MccA can be used as a C-terminal tag for adenylation of proteins in vivo and in vitro. (A) The structure of the fusion protein containing MBP fused to MccA through a linker containing a factor Xa cleavage site is schematically shown. Factor Xa cleavage results in generation of an ISEFGGGGMRTGNAN peptide with an Mw of 1,466.7. (B) Left, the MBP-MccA fusion protein was purified and combined with MccB in the presence or in the absence of ATP under conditions favoring adenylation. Reaction mixtures were treated with factor Xa and subjected to MALDI-MS. Right, MBP-MccA protein was expressed in cells with or without MccB, purified, and subjected to factor Xa treatment, and products were analyzed by MALDI-MS. (C) Purified MBP-MccA was incubated with MccB in the presence or in the absence of [α-32P]ATP. Reaction products were resolved by SDS-PAGE and stained with Coomassie blue (left) (bands corresponding to MBP-MccA and MccB are indicated). The leftmost lane contains molecular mass markers. An autoradiograph of the gel is shown on the right.