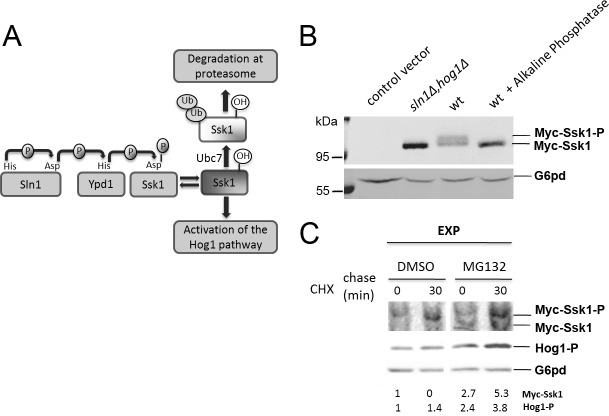
Fig 2. Detection of phosphorylated and unphosphorylated Ssk1.
(A) Schematic diagram of Sln1 branch. Under normal osmotic conditions, Sln1 is autophosphorylated by an intrinsic His kinase activity. The phosphate group is transferred to Ssk1 via a phosphorelay system that involves the transfer protein Ypd1. Phosphorylated Ssk1 is unable to bind Ssk2/Ssk22 and keeps the pathway inactive. During osmotic stress Sln1 kinase activity is inhibited and unphosphorylated Ssk1 activates the Hog1 pathway and is ubiquitinated via Ubc7 and degraded by the proteasome. (B) Wild-type (wt) and sln1Δhog1Δ cells transformed with Myc-Ssk1 plasmid (pMV06) or wt cells containing the pRS415 vector (control) were grown as indicated. Cell extracts were analyzed by SDS-PAGE followed by immunoblotting with anti-Myc antibody. The presence of phosphorylated Ssk1 (Myc-Ssk1-P) was determined by shift of electrophoretic mobility after treatment with alkaline phosphatase CIP performed according to manufacturer’s instructions. Glucose-6-phosphate dehydrogenase (G6pd) was detected as the loading control. (C) Effects of proteasome inhibition by MG132 on degradation of Myc-Ssk1 and phosphorylation of Hog1. Wild-type yeast cells expressing Myc-Ssk1 were exponentially grown (EXP) and treated with the proteasome inhibitor MG132 or DMSO (control), containing cycloheximide to stop protein synthesis. The degradation of unphosphorylated Ssk1 was analyzed on 7% Phos-tag gel containing 50μM Phos-tag acrylamide followed by immunoblotting with anti-Myc antibody. Levels of phosphorylated Hog1 were analyzed on normal SDS-PAGE followed by immunoblotting with anti-phospho-p38 antibody. Glucose-6-phosphate dehydrogenase (G6pd) was detected as the loading control. Levels of phosphorylated Hog1 and unphosphorylated Ssk1 were normalized using G6pd levels and then the relative quantification was normalized using the DMSO sample at time 0.