Fig 6. Structural representation of the five residues on the open state crystal structure.
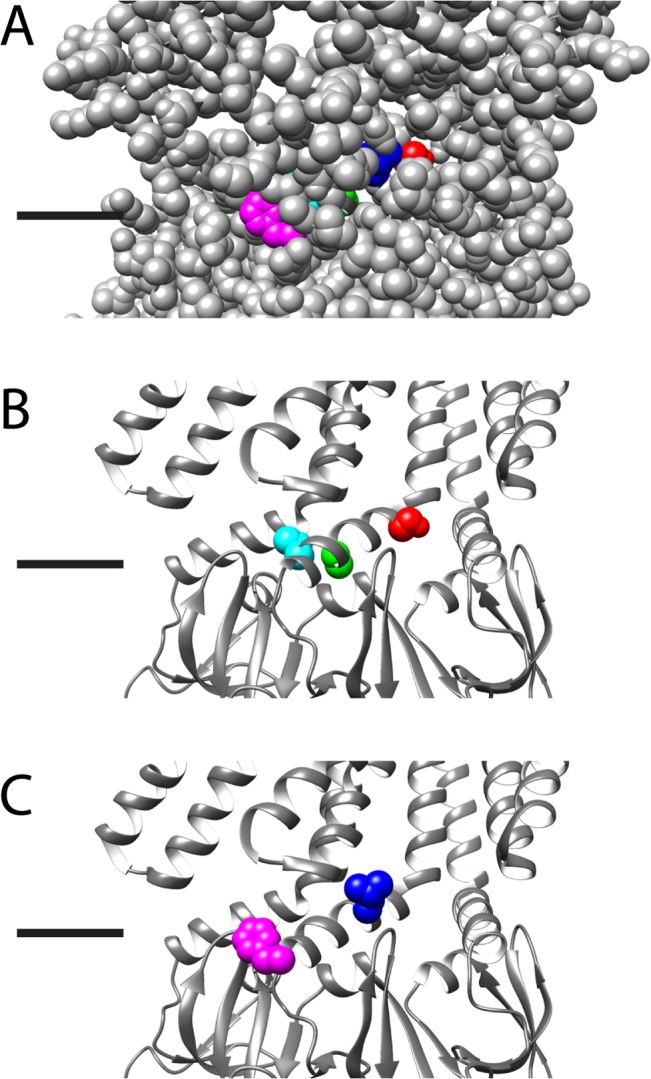
Structural representation of the residues (S114, red; L118, blue; A120, lime green; L123, aqua; F127, magenta) on the open state crystal structure, black lines indicate the predicted location of the lipid headgroups, for clarity three adjacent subunits are shown and the residues are shown as spacefilling in color on the center subunit only. A) All residues are shown as spacefill, showing that L118 and F127 are “lipid exposed”, or visible from the exterior of the protein, while S114, A120, and L123 are buried. B) The protein backbone is shown as a ribbon, and the buried residues are shown in CPK. These residues when mutated are predicted to alter the protein-protein interactions leading to kinetic changes. C) The protein backbone is shown as a ribbon, and the lipid-exposed residues are shown in CPK. As these residues are predicted to be on the surface of the protein it is likely that their interactions with the lipids lead to alteration of channel behavior.
