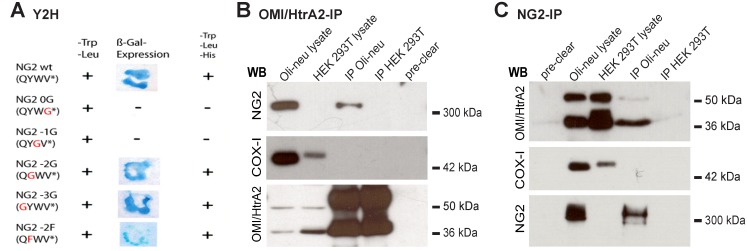
Fig 1. Binding of the NG2 C-terminus to the PDZ-domain containing protein OMI/HtrA2.
A) Yeast two hybrid screen: Yeast cells were transformed with the serine protease OMI/HtrA2 and positive clones defined by ß-Gal-expression. Selection was on Trp-, Leu- and His-deficient media. The NG2 PDZ-binding motif and different mutants (red letters) of this domain were tested for binding. B) Lysates of Oli-neu cells and HEK-293T cells (as a control) were immunoprecipitated with pcOMI/HtrA2 antibody and immunoblotted as indicated. Endogenous NG2 is coimmunoprecipitated with the OMI/HtrA2-IP. As a negative control, the mitochondrial membrane protein COX-1 was analysed. C) Lysates of Oli-neu cells and HEK-293T cells were immunoprecipitated with monoclonal NG2 antibody and immunoblotted as indicated. Endogenous OMI/Htra2, mainly the processed 37 kDa form, is coimmunoprecipitated with the immunoprecipitated NG2-IP. As a negative control, the mitochondrial membrane protein COX-1 was analysed. In the pre-clear fraction, beads without the antibodies served as additional controls.