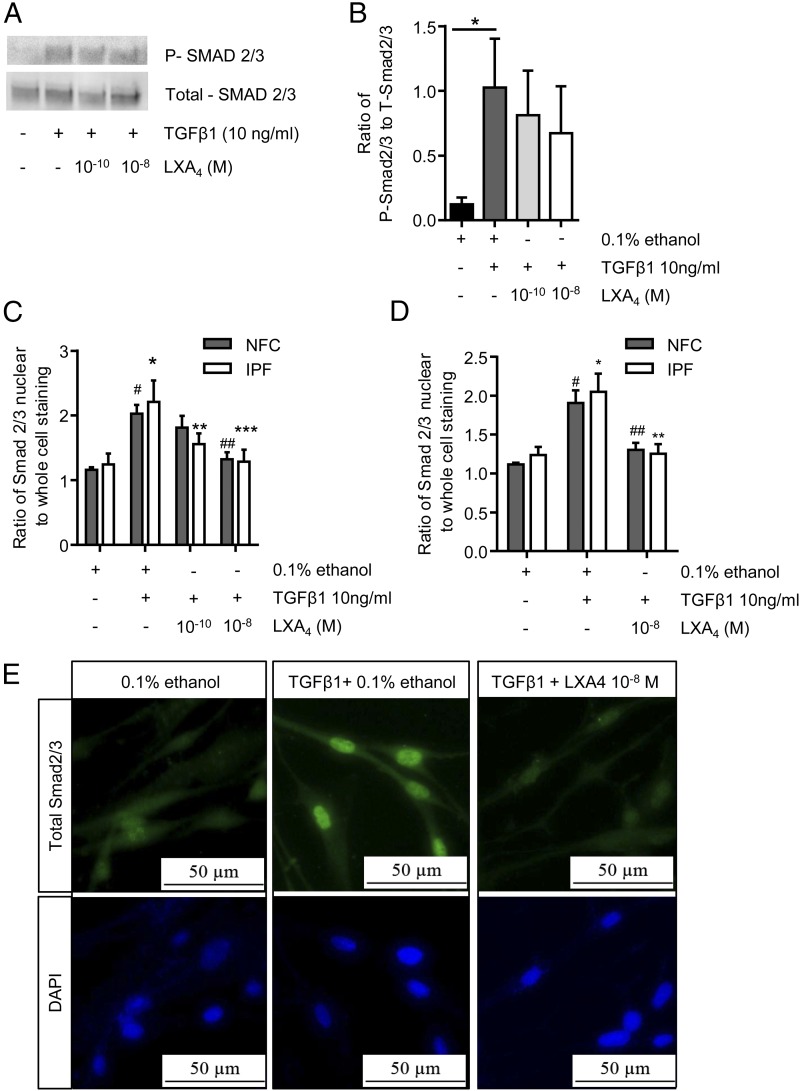
FIGURE 7.
LXA4 does alter Smad2/3 phosphorylation but exerts its effect through inhibition of Smad2/3 translocation to the nucleus. (A and B) Using Western blot analysis, we investigated the effect of LXA4 on TGF-β1–induced phosphorylation of Smad2/3; a representative image from an NFC donor is shown. TGF-β1 significantly increased Smad2/3 phosphorylation in HLMFs (*p = 0.0110; NFC, n = 2; and IPF, n = 3). However, LXA4 at 10−10 and 10−8 mol had no significant effect on the TGF-β1–dependent increase in Smad2/3 phosphorylation in either NFC- or IPF-derived HLMFs. (C) Immunofluorescent analysis of total Smad2/3 nuclear translocation showed a significant increase in both NFC (n = 5) and IPF (n = 4) HLMFs (#p = 0.0007, *p = 0.0007, respectively). This TGF-β1–dependent increase in total nuclear translocation was significantly and dose dependently attenuated by LXA4 (two-way ANOVA corrected by Sidak multiple-comparison test; NFC, 10−8 mol, ##p = 0.0051; IPF, 10−10 mol, **p = 0.0208 and 10−8 mol, ***p = 0.0011). (D) In both NFC- (n = 6) and IPF-derived (n = 6) HLMFs, TGF-β1–dependent total Smad2/3 nuclear translocation was attenuated by LXA4 10−8 mol (two-way ANOVA corrected by Sidak multiple-comparison test; NFC, #p = 0.0003, ##p = 0.00037; IPF, *p = 0.0002, **p = 0.0003). (E) Representative immunofluorescent images from an NFC donor demonstrating the increased nuclear translocation of total Smad2/3 following TGF-β1 stimulation and the inhibition of this by LXA4 (10−8 mol).