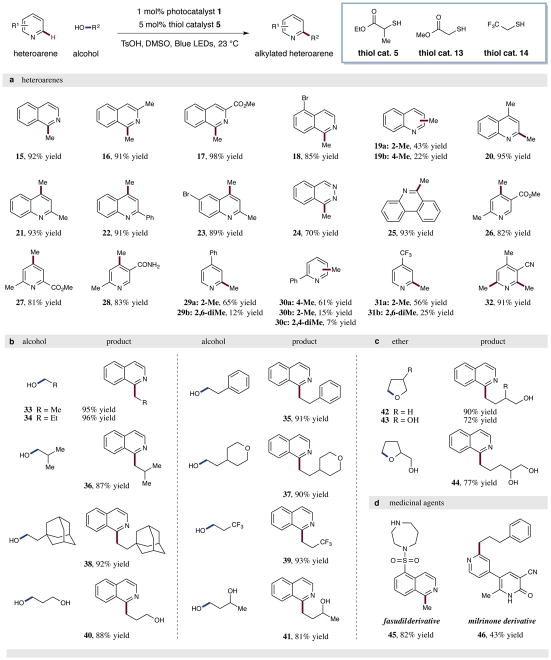
Figure 3. Substrate scope for the alkylation of heteroaromatic C–H bonds with alcohols via the dual photoredox organocata-lytic platform.
A broad range of heteroaromatics and alcohols are efficiently coupled to produce alkylated heterocycles under the standard reaction conditions (top, generalized reaction). (a) A variety of isoquinolines, quinolines, phthalazines, phenanthridines, and pyridines are efficiently methylated using methanol as the alkylating reagent. (b) A diverse selection of alcohols serve as effective alkylating agents in this dual catalytic protocol. (c) Ethers are also amenable to the transformation – the products are the corresponding ring opened alcohols. (d) Two pharmaceuticals, fasudil and milrinone, can be alkylated using this protocol, demonstrating its utility in late-stage functionalization. Isolated yields are indicated below each entry. See Supplementary Information for experimental details.