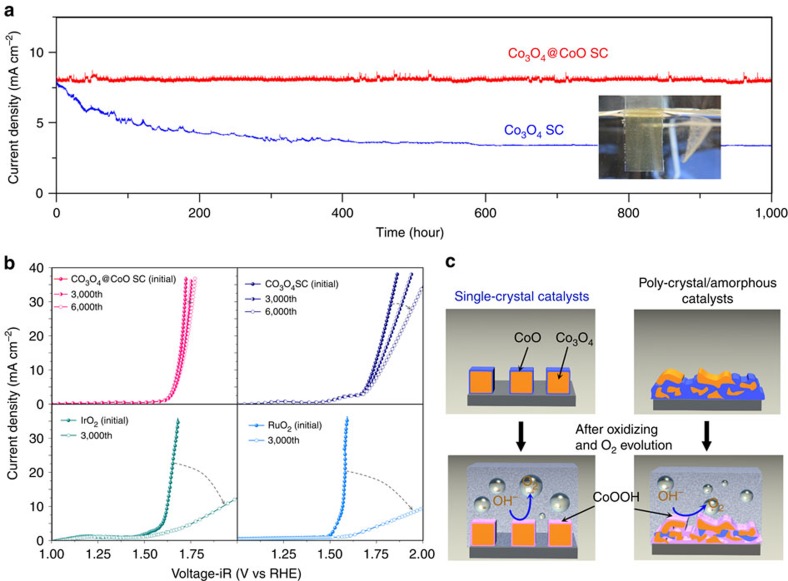
Figure 4. Stability test.
(a) Current versus time data on a Co3O4@CoO SC electrode and Co3O4 SC electrode in 0.5 M KOH (pH=13.6) for 1,000 h (>40 days) at a constant potential of 1.85 V (versus RHE), in which no significant current throughput decay was observed as compared to Co3O4 SC electrode. (b) OER polarization curves of Co3O4@CoO SC, Co3O4 SC, IrO2, and RuO2 catalysts subjected to continual potential cycling between 1.0 and 2.0 V (versus RHE) in O2-saturated KOH (0.5 M) for 3,000 (and 6,000) cycle measurements; the Co3O4@CoO SC retained activity, unlike the IrO2 and RuO2 control catalysts, which failed to achieve the initial current density. All measurements were performed using rotating disk electrode (RDE) voltammetry upon a glassy carbon (GC) disk in desired electrolyte. (c) Schematic representation of structural transformation within single-crystal and amorphous/polycrystal electrocatalysts, indicating that the formation of more active/oxidized phases may generate structural stress and/or voids and weaken the stability of materials. The CoO layer that eliminated the substantial volume change during the OER provided excellent protection for the Co3O4 nanocubes.