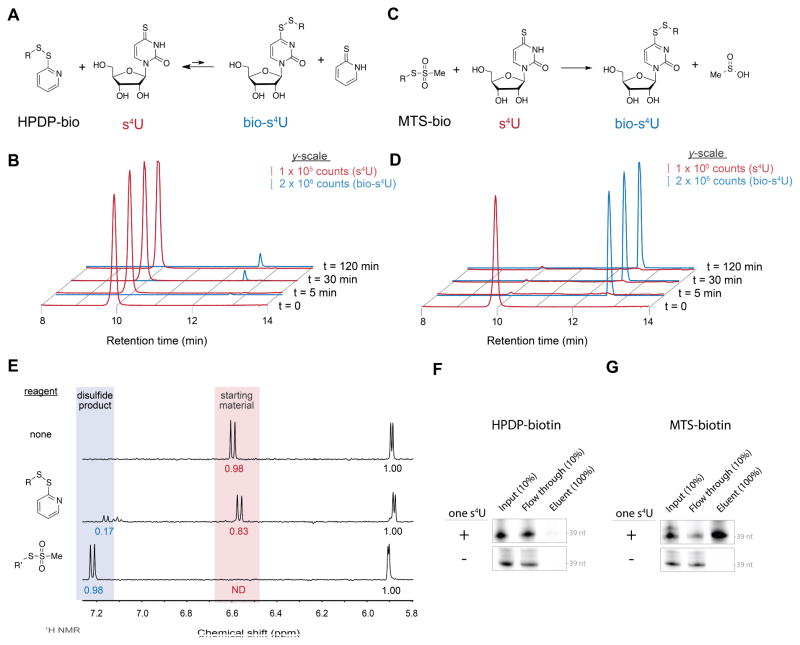
Figure 1. Efficient formation of disulfides with s4U via MTS chemistry.
(A) s4U disulfide exchange with HPDP-biotin. (B) LC-MS extracted ion chromatograms of s4U (red) and biotin-s4U (blue) for HPDP-biotin at the indicated reaction times. (C) s4U disulfide exchange with MTS-biotin. (D) LC-MS chromatograms as in (B). (E) Downfield 1H NMR spectra of (top) s4U alone, (center) s4U reacted with 3-[2-Pyridyldithio]propionyl hydrazide (PDPH), an HPDP-like disulfide, and (bottom) methyl-MTS. Peaks for the starting material (red shading) and products (blue shading) were integrated and normalized to sum of the anomeric protons of s4U and its products (5.9 ppm). For full spectra, see Figure S1A–C. (F, G) Enrichment of a singly-thiolated 39-nt RNA by (F) HPDP-biotin or (G) MTS-biotin. Fluorescently labeled 39-nt RNAs with or without a single s4U were biotinylated with the indicated reagent and enriched on streptavidin beads, followed by urea-PAGE and fluorescence imaging. See also Figure S1.