Abstract
Purpose of review
Cushing syndrome caused by cortisol-producing adrenal adenomas is a rare condition, associated with high morbidity due to weight gain, diabetes mellitus, osteoporosis, hypertension, muscle weakness, mood disturbance, etc. The first gene to be identified as causative of Cushing syndrome was PRKAR1A. We present an update on protein kinase A (PKA) defects and Cushing syndrome.
Recent findings
The cyclic AMP-dependent PKA catalytic subunit alpha (PRKACA) hotspot point mutation (c.617A>C [p.Leu206Arg]), leading to an increase of basal protein kinase A (PKA) activity, and formation of cortisol-producing adenoma has been frequently shown to cause the most common form of Adrenocorticotropic hormone-independent Cushing syndrome.
Summary
Somatic PRKACA mutations have been found in up to 50% of patients with adrenal adenomas. Germline PRKACA amplification was also seen in bilateral adrenal hyperplasias. PRKACA activation was associated with higher cortisol levels, smaller tumor size and overt Cushing syndrome. This breakthrough is expected to improve our understanding of how PKA defects lead to Cushing syndrome and may spearhead the development of new, molecularly designed therapies.
Keywords: PRKAR1A, PRKACA, PRKACB, PKA, Cushing syndrome, cortisol-producing adrenal adenoma, adrenal tumor
Introduction
Adrenal tumors are found increasingly more commonly due to the number of imaging studies obtained in the general population [1]. A small fraction of these tumors are producing hormones. Endogenous overproduction of cortisol by the adrenal adenomas may cause Cushing syndrome, associated with significant morbidity [2]. Adrenal Cushing syndrome is often missed, given that some of the patients have a subclinical or even cyclical Cushing syndrome. Overt Cushing syndrome is diagnosed in patients with significant metabolic abnormalities, such as weight gain, diabetes mellitus, hypertension, osteoporosis, etc.
Genetic mutations, causing cortisol-producing tumors have been suspected for years, but only few genetic defects had been discovered until recently. Patients with McCune-Albright syndrome have somatic mutations in Guanine Nucleotide Binding Protein, Alpha Stimulating (GNAS)1 in their adrenal glands causing cortisol overproduction due to a unique form of bilateral adrenocortical hyperplasia (BAH) or single adenomas. Cushing syndrome caused by primary pigmented nodular adrenocortical disease (PPNAD) is due to mutations in cyclic AMP (cAMP)-dependent protein kinase (PKA) type 1 alpha regulatory subunit (PRKAR1A) [3]. Recently, mutations in the armadillo repeat containing 5 (ARMC5), a tumor-suppressor gene, were found to be the cause of primary macronodular adrenal hyperplasia (PMAH), formerly known as Adrenocorticotropic hormone-independent macronodular adrenal hyperplasia (AIMAH) [4-8]. Finally, phosphodiestarases (PDE) PDE8B and PDE11A may also play an important role in the formation of cortisol-producing adenomas [9-11] or even cancer [12].
Cyclic AMP signaling pathway
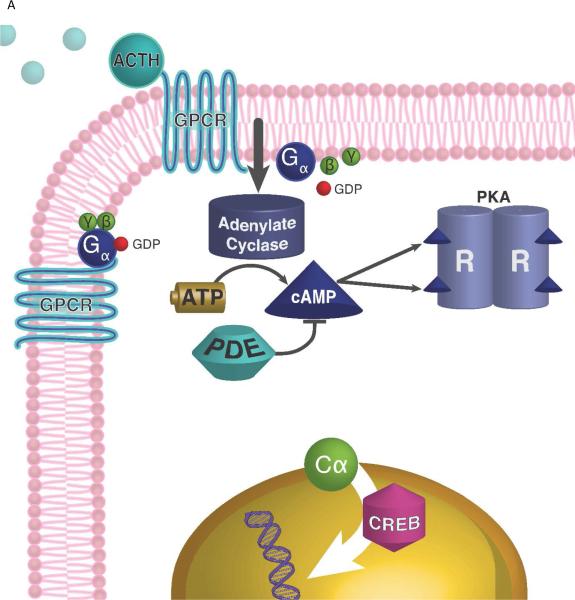
The cAMP/protein kinase A (PKA) pathway is crucial for the function of the adrenal gland [13, 14] (Figure 1). Corticotropin (ACTH) binds to its G protein-coupled transmembrane receptor (MC2R), leading to the synthesis of cAMP by adenylate cyclase [15]. cAMP, acting as a secondary messenger, targets tetramer PKA. The latter is a cAMP-dependent serine-kinase, consisting of two regulatory (with PRKAR1A being the main one) and two inactive catalytic subunits. cAMP binds to the regulatory subunits, dissolves the tetramer, thus enabling catalytic subunits to phosphorylate a number of targets, and activate transcription of the genes.
Figure 1.
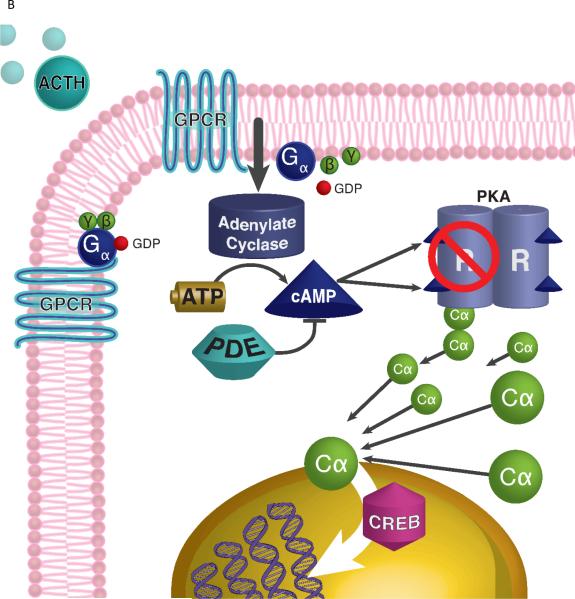
Protein Kinase A signaling and adrenal Cushing syndrome. A. ACTH binds to its 7-transmembrane G-protein coupled receptor (GPCR), which activates Gsα protein and stimulates adenylate cyclase to generate cAMP (from ATP). PKA is a tetrameric enzyme, composed by 2 regulatory (R) and 2 catalytic (C) subunits, and is activated by an increase of the intracellular cAMP concentration: cAMP molecules bind to the R subunits which then set free the C subunits, resulting in phosphorylation of transcription factor cAMP response element binding protein (CREB) and other target molecules. Phosphodiesterases (PDEs), like PDE11A and PDE8B, bind cAMP and decrease its levels. B. Increased PKA activity in the setting of somatic PRKACA mutations in patients with cortisol-producing adenomas is due to the lack of the mutant PRKACA's binding to the R subunits, or due to excess C subunits caused by copy number gain of the gene coding for it.
The role of the cAMP pathway in adrenal tumors associated with Cushing syndrome has been repeatedly shown. For example, inactivating mutations in PRKAR1A in patients with PPNAD lead to inactivation of R1α, which allows Cα to be uninhibited [16], resulting in PKA activation.
The PRKAR1A gene, Carney complex, and PPNAD
Carney complex is a multiple endocrine neoplasia syndrome, inherited in an autosomal dominant pattern [17, 18]. Patients have PPNAD, as well as other endocrine neoplasms, such as pituitary adenoma and/or hyperplasia, thyroid and gonadal tumors. It is also associated with many non-endocrine lesions, such as spotty skin pigmentation (also known as “lentiginosis”), myxomas and schwannomas [17, 19]. PPNAD, which causes an often indolent form of adrenal Cushing syndrome, is the most common endocrine manifestation of Carney complex. Most of the affected patients present as children or young adults. Patients have a rather unique “paradoxical” rise of cortisol after the high-dose dexamethasone part of the Liddle's test [18, 20].
There are two genetic loci (17q22-24 and 2p16), which are associated with this complex. Mutations in the PRKAR1A gene were first recognized in families with linkage to the 17q22-24 locus [21]. Approximately two thirds of the patients affected by Carney complex have PRKAR1A mutations, while no gene has been identified at the 2p16 locus to-date [22]. PRKAR1A is likely to be a tumor suppressor gene, since the allelic loss of the wild-type allele is often seen in patients with Carney complex [3, 21]. To date more than 126 PRKAR1A mutations have been described. A publicly available database is available online, and continuously updated by our team (http://prkar1a.nichd.nih.gov/hmdb/mutations.html). Most of these mutations encompass small deletions and single base substitutions, or open reading frame rearrangements, with a few large deletions reported [23]. Importantly, an association between Carney complex and adrenal cancer, was recently described in patients with PRKAR1A mutations [24, 25]. Other cancerous associations, such as hepatocellular carcinoma [26] and pancreatic malignancies have been reported [27].
PRKACA defects and Cushing syndrome
Recently, Beuschlein et al. identified somatic activating mutations of the main catalytic subunit of PKA, PRKACA, in unilateral cortisol-producing adrenal tumors, causing overt Cushing syndrome [28]; this discovery was confirmed by others [29-34]. These groups performed whole exome DNA sequencing of the available tumors and leukocyte DNA, and identified a recurring hotspot point PRKACA mutation (c.617A>C, also known as c.617T>G), resulting in arginine substitution of amino acid 206 (Leu206Arg).
Beuschlein at al. studied 139 patients with adrenal adenomas, adrenocortical carcinomas and ACTH-independent primary adrenal hyperplasias [28]. These patients were screened for PDE8B, PDE11A, PRKAR1A mutations and were found to be negative. The researchers identified mutations in PRKACA in 8 of the 10 originally screened unilateral cortisol-producing adenomas, with a majority (7 patients) having the c.617A>C, p.Leu206Arg mutation, whereas one had the insertion located at c.595_596CAC, Leu199_Cys200insTrp. The p.Leu206Arg mutation is located in a highly conservative core of the interaction between the regulatory (RIIβ) and catalytic subunits of PKA. Importantly, these investigators described the clinical phenotype of these patients: 37 % of the studied cohort had overt Cushing syndrome due to a unilateral cortisol-producing adenoma with a PRKACA mutation. These patients had a higher index of disease severity, as shown by increased urinary free cortisol and late-night serum cortisol levels. These findings were correlated with expression levels of the steroidogenic enzymes that were higher in tissues with PRKACA mutations. Patients with germline copy number gain of the PRKACA locus on chromosome 19p had BAH.
Shortly after the initial report, we described in detail the three pathologic phenotypes of BAH of the previously reported [28] five patients with germline PRKACA copy number gain. Three patients had a disease that looked like PPNAD plus extranodular cortical atrophy and mild intra- and extracapsular extension of adrenocortical cells, whereas others had cortical hyperplasia and capsular and extracapsular micronodular cortical hyperplasia [34].
The described hotspot mutations [28] may interfere with the creation of a stable PKA molecule and render the mutant Cα subunits constitutively active [35]. Cao and collaborators performed whole exome sequencing of 49 tumors and RNA sequencing of 44 tumors, including cortisol-producing adenomas, carcinomas and AIMAH [29]. They identified an even higher rate of somatic PRKACA mutations: 69.2% (27 out of 39). They published a hotspot c.617T>G, resulting in Leu205Arg substitution. It should be noted that this is the same mutation that was reported by Beuschlein et al. [28]: apparently, Cao et al. used a different amino acid numbering system, where initiating methionine is counted as residue zero (and not one), thus reporting Leu205Arg vs. Leu206Arg [36]. Interestingly, they found no statistical significant differences in serum cortisol, plasma ACTH, and urinary free cortisol levels, when compared to the patients without PRKACA mutations. The researchers performed functional studies (gain of function mutation in 293T cells), and found that overexpression of Leu205Arg mutants increases phosphorylation of PKA derivatives relative to the wild type [29]. In particular, the PRKACA Leu205Arg mutation induced phosphorylation of the cAMP response element binding protein (CREB), confirming the hypothesis that Leu205Arg mutation may enhance PKA activity [28].
In the same issue of the journal Science, Sato and colleagues independently reported finding the identical Leu206Arg hotspot mutation in PRKACA [32], as the cause of Cushing syndrome in patients with adrenal tumors. The researchers performed whole exome sequencing on eight adrenal tumors, and found 50% of them had the mentioned mutations. Moreover, they screened 57 follow-up cases and found that 24 of them had the PRKACA Leu206Arg somatic mutations. The affected patients had a smaller tumor size (p=0.00005) and higher levels of serum cortisol after 1 mg dexamethasone suppression test (p=0.0026) [32], reporting similar findings to others [31]. They also expressed wild-type PRKACA and the Leu206Arg in Human Embryonic Kidney 293 cells in which they showed that the Leu206Arg PRKACA mutant did not interact with the PKA regulatory subunits. The wild-type and mock PRKACA-transduced cells demonstrated enhanced PKA activity and increased CREB phosphorylation, irrespective of forskolin treatment [32]. Sato et al also detected GNAS mutations in 16.9% of the studied cohort.
Goh et al., reported the results of exome sequencing of cortisol-producing tumors from 25 patients (22 with adrenocortical adenoma and 3 with adrenocortical carcinoma) [31]. They found PRKACA heterozygous somatic mutations (c.617A>C [p.Leu206Arg]) in 6 patients. Similarly to the original study [28], they discovered a higher steroidogenic enzymatic activity in the adrenal tissue with PRKACA mutations, thus causing tumor development and endogenous Cushing syndrome. Patients with mutant adrenal adenomas were younger and had smaller tumors, associated with overt Cushing syndrome.
Di Dalmazi and colleagues investigated 149 frozen tumor samples from nine European medical centers, paired with leucocyte DNA and patients clinical and biochemical data, when available [30]. Similarly to the previous investigators, they found mutations of exon 7 of PRKACA gene in 34% studied samples, associated with Cushing syndrome. In addition to previously described missense mutation c.617A>C (p.Leu206Arg) (18 out of 22 patients), they found two novel mutations in another 4 patients (c.600_601insGTG/p.Cys200_Gly201insVal and c.639C>G+c.638_640insATTATCCTGAGG/p.Ser213Arg+p.Leu212_Lys214insIle-Ile-Leu-Arg). No germline PRKACA mutations were identified. The authors found higher levels of serum cortisol after dexamethasone testing, and smaller size of cortisol-producing adenomas.
Recently, Nakajima et al. screened tumors of 13 patients in Japan, and found recurrent somatic mutations of the PRKACA gene, p.L206R (c.617T>G) in 23% of patients with overt Cushing syndrome [33].
The PRKACB gene and Carney complex
We recently found that genomic amplification of the PRKACB locus may lead to Carney Complex without any PRKAR1A mutations [37]. Forlino et al. described a single case of a 19-year-old female patient presenting with acromegaly and lentigines, who also had myxomas, but no Cushing syndrome. This patient had somatic copy number gain of the chromosome 1p31.1 PRKACB locus, leading to an increase of the PKA catalytic subunit Cβ expression and higher PKA activity in the patient's cells. A mouse model, carrying a transgene for human PRKACB, had an increase in growth hormone secretion [37].
Conclusions
Somatic mutations in PRKACA may be a frequent cause of Cushing syndrome in patients with adrenal adenomas, while BAH may be caused by PRKAR1A or PRKACA defects. PRKACA mutations that lead to Cushing syndrome prevent the binding of the catalytic to the regulatory subunits, thus causing constitutive and cAMP-independent activation of PKA, and eventually, “autonomous” overproduction of cortisol. It is possible that larger tumors produce cortisol less efficiently, whereas smaller tumors may be more steroidogenic. This exciting discovery has been well covered in the scientific literature [36, 38-44], is expected to lead to a number of questions: Do PRKACA gene mutations cause tumors and cancers in other organs (breast, colon, liver, pituitary, etc.), since cAMP/PKA is instrumental in virtually every tissue in the human body? Does mutation in other PKA catalytic subunits (Cβ, Cγ, and PRKX) cause adrenal adenomas and/or hyperplasia? Moody and colleagues reported increased PRKACA expression in trastuzumab-resistant breast cancer, suggesting that the cAMP/PKA pathway may be stimulated in some breast cancers [45]. Fibrolamellar hepatocellular carcinoma was found to be due to a recurrent DnaJ homolog subfamily B member 1 (DNAJB1)-PRKACA chimeric transcript [46]. Parathyroid hormone receptor (PTHR1) and parathyroid related protein (PTHrP) may promote tumor invasion and proliferation in osteosarcoma, via enhanced PKA signaling [47]. Vitali et al. showed that cAMP surges DNA synthesis and cyclin D1 expression in somatotropinomas, and abolishes in prolactinomas and non-functioning pituitary tumors [48].
The new genetics of Cushing syndrome may assist clinicians to provide appropriate counseling and in their decision-making regarding medical and/or surgical intervention. These findings may also lead to the development of individualized pharmacological treatment(s) for Cushing syndrome and other disease associated with PKA defects.
Key points.
Somatic mutations of the main catalytic subunit of PKA, a serine-threonine kinase, PRKACA, may cause cortisol-producing adenomas and Adrenocorticotropic hormone-independent Cushing syndrome.
Germline duplications of the PRKACA may result in bilateral adrenal hyperplasia.
Patients with PRKACA defects may have smaller tumors and more severe forms of Cushing syndrome.
The cAMP/PKA pathway is a potential target for molecularly designed therapies of Cushing syndrome.
Acknowledgements
We thank Diane Cooper, MSLS, NIH Library, for providing assistance in writing this manuscript. We thank Jeremy Swan and Nichole Jonas for providing assistance with images.
Financial support and sponsorship
This research was supported by the Intramural Research Program of Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (NIH).
Abbreviations
- PRKACA
protein kinase catalytic subunit alpha
- PRKACB
protein kinase catalytic subunit beta
- PRKAR1A
protein kinase type 1 alpha regulatory subunit
- PPNAD
primary pigmented nodular adrenal dysplasia
- cAMP
cyclic AMP
- ARMC5
armadillo repeat containing 5
- AIMAH
ACTH-independent macronodular adrenal hyperplasia
- BAH
bilateral adrenocortical hyperplasia
- PMAH
primary macronodular adrenal hyperplasia
- PDE
phosphodiesterase
- PKA
protein kinase A
- CREB
response element binding protein
- PRKACB
protein kinase catalytic subunit beta
- DNAJB1
DnaJ homolog subfamily B member 1
- PTHR1
Parathyroid hormone receptor
- PTHrP
parathyroid related protein
Footnotes
Conflicts of interest
The authors have no conflicts of interest.
References and recommended reading
- 1.Young WF. The Incidentally Discovered Adrenal Mass. N. Engl. J. Med. 2007;356:601–610. doi: 10.1056/NEJMcp065470. [DOI] [PubMed] [Google Scholar]
- 2.Stratakis CA. Cushing syndrome caused by adrenocortical tumors and hyperplasias (corticotropin- independent Cushing syndrome). Endocr Dev. 2008;13:117–132. doi: 10.1159/000134829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirschner LS, Sandrini F, Monbo J, et al. Genetic heterogeneity and spectrum of mutations of the PRKAR1A gene in patients with the carney complex. Hum. Mol. Genet. 2000;9:3037–3046. doi: 10.1093/hmg/9.20.3037. [DOI] [PubMed] [Google Scholar]
- 4.Elbelt U, Trovato A, Kloth M, et al. Molecular and Clinical Evidence for an ARMC5 Tumor Syndrome: Concurrent Inactivating Germline and Somatic Mutations are Associated with both Primary Macronodular Adrenal Hyperplasia and Meningioma. J. Clin. Endocrinol. Metab. 2014:jc20142648. doi: 10.1210/jc.2014-2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gagliardi L, Schreiber AW, Hahn CN, et al. ARMC5 mutations are common in familial bilateral macronodular adrenal hyperplasia. J. Clin. Endocrinol. Metab. 2014;99:E1784–1792. doi: 10.1210/jc.2014-1265. [DOI] [PubMed] [Google Scholar]
- 6.Alencar GA, Lerario AM, Nishi MY, et al. ARMC5 mutations are a frequent cause of primary macronodular adrenal Hyperplasia. J. Clin. Endocrinol. Metab. 2014;99:E1501–1509. doi: 10.1210/jc.2013-4237. [DOI] [PubMed] [Google Scholar]
- 7*.Faucz FR, Zilbermint M, Lodish MB, et al. Macronodular adrenal hyperplasia due to mutations in an armadillo repeat containing 5 (ARMC5) gene: a clinical and genetic investigation. J. Clin. Endocrinol. Metab. 2014;99:E1113–1119. doi: 10.1210/jc.2013-4280. [First NIH report of patients with primary macronodular adrenal hyperplasia, who found to have germline and somatic ARMC5 gene mutations, associated with overt Cushing syndrome.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Assie G, Libe R, Espiard S, et al. ARMC5 mutations in macronodular adrenal hyperplasia with Cushing's syndrome. N. Engl. J. Med. 2013;369:2105–2114. doi: 10.1056/NEJMoa1304603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boikos SA, Horvath A, Heyerdahl S, et al. Phosphodiesterase 11A expression in the adrenal cortex, primary pigmented nodular adrenocortical disease, and other corticotropin-independent lesions. Horm. Metab. Res. 2008;40:347–353. doi: 10.1055/s-2008-1076694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horvath A, Boikos S, Giatzakis C, et al. A genome-wide scan identifies mutations in the gene encoding phosphodiesterase 11A4 (PDE11A) in individuals with adrenocortical hyperplasia. Nat. Genet. 2006;38:794–800. doi: 10.1038/ng1809. [DOI] [PubMed] [Google Scholar]
- 11.Horvath A, Mericq V, Stratakis CA. Mutation in PDE8B, a cyclic AMP-specific phosphodiesterase in adrenal hyperplasia. N. Engl. J. Med. 2008;358:750–752. doi: 10.1056/NEJMc0706182. [DOI] [PubMed] [Google Scholar]
- 12.Libe R, Fratticci A, Coste J, et al. Phosphodiesterase 11A (PDE11A) and genetic predisposition to adrenocortical tumors. Clin. Cancer Res. 2008;14:4016–4024. doi: 10.1158/1078-0432.CCR-08-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Joussineau C, Sahut-Barnola I, Levy I, et al. The cAMP pathway and the control of adrenocortical development and growth. Mol. Cell. Endocrinol. 2012;351:28–36. doi: 10.1016/j.mce.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott JD. Cyclic nucleotide-dependent protein kinases. Pharmacol. Ther. 1991;50:123–145. doi: 10.1016/0163-7258(91)90075-w. [DOI] [PubMed] [Google Scholar]
- 15.Das R, Esposito V, Abu-Abed M, et al. cAMP activation of PKA defines an ancient signaling mechanism. Proc. Natl. Acad. Sci. U. S. A. 2007;104:93–98. doi: 10.1073/pnas.0609033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meoli E, Bossis I, Cazabat L, et al. Protein kinase A effects of an expressed PRKAR1A mutation associated with aggressive tumors. Cancer Res. 2008;68:3133–3141. doi: 10.1158/0008-5472.CAN-08-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carney JA, Gordon H, Carpenter PC, et al. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine (Baltimore) 1985;64:270–283. doi: 10.1097/00005792-198507000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Stratakis CA, Kirschner LS, Carney JA. Clinical and molecular features of the Carney complex: diagnostic criteria and recommendations for patient evaluation. J. Clin. Endocrinol. Metab. 2001;86:4041–4046. doi: 10.1210/jcem.86.9.7903. [DOI] [PubMed] [Google Scholar]
- 19.Rothenbuhler A, Stratakis CA. Clinical and molecular genetics of Carney complex. Best practice & research. Clinical endocrinology & metabolism. 2010;24:389–399. doi: 10.1016/j.beem.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Stratakis CA, Sarlis N, Kirschner LS, et al. Paradoxical response to dexamethasone in the diagnosis of primary pigmented nodular adrenocortical disease. Ann. Intern. Med. 1999;131:585–591. doi: 10.7326/0003-4819-131-8-199910190-00006. [DOI] [PubMed] [Google Scholar]
- 21.Kirschner LS, Carney JA, Pack SD, et al. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat. Genet. 2000;26:89–92. doi: 10.1038/79238. [DOI] [PubMed] [Google Scholar]
- 22.Stratakis CA, Carney JA, Lin JP, et al. Carney complex, a familial multiple neoplasia and lentiginosis syndrome. Analysis of 11 kindreds and linkage to the short arm of chromosome 2. J. Clin. Invest. 1996;97:699–705. doi: 10.1172/JCI118467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horvath A, Bossis I, Giatzakis C, et al. Large deletions of the PRKAR1A gene in Carney complex. Clin. Cancer Res. 2008;14:388–395. doi: 10.1158/1078-0432.CCR-07-1155. [DOI] [PubMed] [Google Scholar]
- 24.Anselmo J, Medeiros S, Carneiro V, et al. A large family with Carney complex caused by the S147G PRKAR1A mutation shows a unique spectrum of disease including adrenocortical cancer. The Journal of Clinical Endocrinology & Metabolism. 2011;97:351–359. doi: 10.1210/jc.2011-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morin E, Mete O, Wasserman JD, et al. Carney complex with adrenal cortical carcinoma. J. Clin. Endocrinol. Metab. 2012;97:E202–206. doi: 10.1210/jc.2011-2321. [DOI] [PubMed] [Google Scholar]
- 26.Gennari M, Stratakis CA, Hovarth A, et al. A novel PRKAR1A mutation associated with hepatocellular carcinoma in a young patient and a variable Carney complex phenotype in affected subjects in older generations. Clin. Endocrinol. (Oxf) 2008;69:751–755. doi: 10.1111/j.1365-2265.2008.03286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaujoux S, Tissier F, Ragazzon B, et al. Pancreatic ductal and acinar cell neoplasms in Carney complex: a possible new association. J. Clin. Endocrinol. Metab. 2011;96:E1888–1895. doi: 10.1210/jc.2011-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28**.Beuschlein F, Fassnacht M, Assie G, et al. Constitutive activation of PKA catalytic subunit in adrenal Cushing's syndrome. N. Engl. J. Med. 2014;370:1019–1028. doi: 10.1056/NEJMoa1310359. [This is a first report of somatic PRKACA mutations and germline PRKACA amplification to be found in patients with adrenal adenomas, and bilateral adrenal hyperplasias, respectively.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29*.Cao Y, He M, Gao Z, et al. Activating hotspot L205R mutation in PRKACA and adrenal Cushing's syndrome. Science. 2014;344:913–917. doi: 10.1126/science.1249480. [Confirmatory report of the role of PRKACA mutations in adrenal Cushing syndrome.] [DOI] [PubMed] [Google Scholar]
- 30*.Di Dalmazi G, Kisker C, Calebiro D, et al. Novel somatic mutations in the catalytic subunit of the protein kinase A as a cause of adrenal Cushing's syndrome: a European multicentric study. J. Clin. Endocrinol. Metab. 2014;99:E2093–2100. doi: 10.1210/jc.2014-2152. [Confirmatory report of the role of PRKACA mutations in adrenal Cushing syndrome.] [DOI] [PubMed] [Google Scholar]
- 31*.Goh G, Scholl UI, Healy JM, et al. Recurrent activating mutation in PRKACA in cortisol-producing adrenal tumors. Nat. Genet. 2014;46:613–617. doi: 10.1038/ng.2956. [Confirmatory report of the role of PRKACA mutations in adrenal Cushing syndrome.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32*.Sato Y, Maekawa S, Ishii R, et al. Recurrent somatic mutations underlie corticotropin-independent Cushing's syndrome. Science. 2014;344:917–920. doi: 10.1126/science.1252328. [Confirmatory report of the role of PRKACA mutations in adrenal Cushing syndrome.] [DOI] [PubMed] [Google Scholar]
- 33*.Nakajima Y, Okamura T, Gohko T, et al. Somatic mutations of the catalytic subunit of cyclic AMP-dependent protein kinase (PRKACA) gene in Japanese patients with several adrenal adenomas secreting cortisol [Rapid Communication]. Endocr. J. 2014;61:825–832. doi: 10.1507/endocrj.ej14-0282. [Confirmatory report of the role of PRKACA mutations in adrenal Cushing syndrome.] [DOI] [PubMed] [Google Scholar]
- 34**.Carney JA, Lyssikatos C, Lodish MB, Stratakis CA. Germline PRKACA amplification leads to Cushing syndrome caused by 3 adrenocortical pathologic phenotypes. Hum. Pathol. 2015;46:40–49. doi: 10.1016/j.humpath.2014.09.005. [This manuscript describes in detail the three pathologic phenotypes of bilateral adrenal hyperplasia in patients with germline amplifications of PRKACA mutations.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calebiro D, Hannawacker A, Lyga S, et al. PKA catalytic subunit mutations in adrenocortical Cushing's adenoma impair association with the regulatory subunit. Nature communications. 2014:5. doi: 10.1038/ncomms6680. [DOI] [PubMed] [Google Scholar]
- 36.Giordano TJ. Genetics: Pinpointing a hotspot in adrenal Cushing syndrome. Nature reviews. Endocrinology. 2014;10:447–448. doi: 10.1038/nrendo.2014.89. [DOI] [PubMed] [Google Scholar]
- 37*.Forlino A, Vetro A, Garavelli L, et al. PRKACB and Carney complex. N. Engl. J. Med. 2014;370:1065–1067. doi: 10.1056/NEJMc1309730. [First report of the role of PRKACB mutations in Carney complex.] [DOI] [PubMed] [Google Scholar]
- 38*.Espiard S, Ragazzon B, Bertherat J. Protein kinase a alterations in adrenocortical tumors. Horm. Metab. Res. 2014;46:869–875. doi: 10.1055/s-0034-1385908. [Review of known protein kinase A alterations causing adrenal tumors.] [DOI] [PubMed] [Google Scholar]
- 39.Kirschner LS. Medicine. A unified cause for adrenal Cushing's syndrome. Science. 2014;344:804–805. doi: 10.1126/science.1254901. [DOI] [PubMed] [Google Scholar]
- 40.Sargent J. Neuroendocrine cancer. An activating hotspot mutation in PRKACA provides clues for adrenal Cushing syndrome therapeutics. Nature reviews. Endocrinology. 2014;10:311. doi: 10.1038/nrendo.2014.57. [DOI] [PubMed] [Google Scholar]
- 41*.Stratakis CA. E pluribus unum? The main protein kinase A catalytic subunit (PRKACA), a likely oncogene, and cortisol-producing tumors. J. Clin. Endocrinol. Metab. 2014;99:3629–3633. doi: 10.1210/jc.2014-3295. [Review of protein kinase A alterations, focusing on cortisol-producing adrenal tumors.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cancer: PKA mutations are associated with Cushing syndrome. Nature reviews. Endocrinology. 2014;10:251–251. [Google Scholar]
- 43.Stratakis CA, Bertherat J, PDE Paris, France: another exciting workshop for cyclic AMP, protein kinase A, and phosphodiesterases. Horm. Metab. Res. 2014. 2013;46:825–826. doi: 10.1055/s-0034-1394418. [DOI] [PubMed] [Google Scholar]
- 44.Bertagna X. Genetics of adrenal diseases in 2014: Genetics improves understanding of adrenocortical tumours. Nature reviews. Endocrinology. 2014 doi: 10.1038/nrendo.2014.215. [DOI] [PubMed] [Google Scholar]
- 45.Moody SE, Schinzel AC, Singh S, et al. PRKACA mediates resistance to HER2-targeted therapy in breast cancer cells and restores anti-apoptotic signaling. Oncogene. 2014:0. doi: 10.1038/onc.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Honeyman JN, Simon EP, Robine N, et al. Detection of a Recurrent DNAJB1-PRKACA Chimeric Transcript in Fibrolamellar Hepatocellular Carcinoma. Science. 2014;343:1010–1014. doi: 10.1126/science.1249484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walkley CR, Walia MK, Ho PW, Martin TJ. PTHrP, its receptor, and protein kinase A activation in osteosarcoma. Molecular & Cellular Oncology. 2014:1. doi: 10.4161/23723548.2014.965624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vitali E, Peverelli E, Giardino E, et al. Cyclic adenosine 3′-5′-monophosphate (cAMP) exerts proliferative and anti-proliferative effects in pituitary cells of different types by activating both cAMP-dependent protein kinase A (PKA) and exchange proteins directly activated by cAMP (Epac). Mol. Cell. Endocrinol. 2014;383:193–202. doi: 10.1016/j.mce.2013.12.006. [DOI] [PubMed] [Google Scholar]