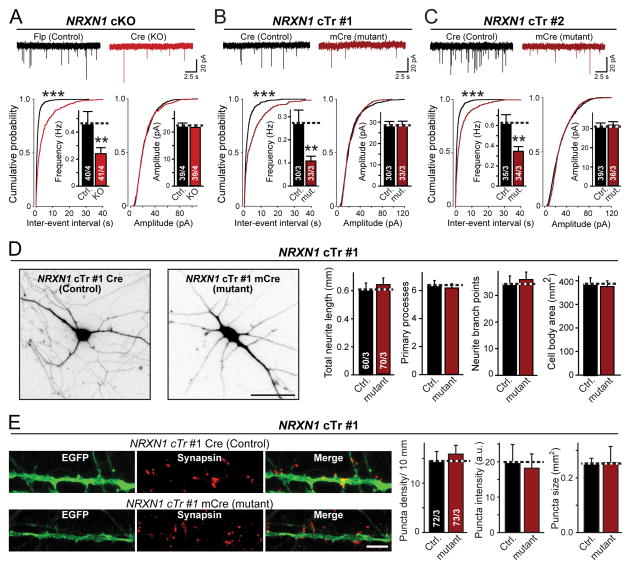
Figure 3. Heterozygous NRXN1 mutation decreases the frequency of spontaneous mEPSCs without altering neuronal morphology or synapse numbers.
(A–C) Heterozygous NRXN1 mutations uniformly decrease the mEPSC frequency approximately 2-fold. Top; representative traces recorded from iN cells derived from the NRXN1 cKO line (A) or the two different cTr lines (B and C); bottom, summary plots and graphs of the frequency (left) and amplitudes (right) of mEPSCs. Quantitations are shown both as cumulative probability plots and as bar diagrams (inserts).
(D) Morphological properties are unchanged in NRXN1-mutant human neurons (left, representative images of control and NRXN1–mutant cTr#1 iN cells that were sparsely infected with lentiviruses expressing tdTomato to label individual neurons [scale bar, 40 μm]; right, quantifications of the total neurite length, number of primary processes, neurite branch points, and cell size in control and NRXN1-mutant iN cells).
(E) Synapse density is unchanged in NRXN1-mutant human neurons (left, representative images of isolated control and mutant cTr#1 iN cells that were co-infected with GFP and stained for the synaptic marker synapsin-1 [scale bar, 5 μm]; right, quantification of the density, staining intensity, and size of synapses in control and mutant iN cells).
Data are means ± SEM; numbers of cells/cultures analyzed are shown in the bars. Statistical significance (**, p<0.01; ***, p<0.001) was evaluated with the Kolmogorov-Smirnov-test (cumulative probability plots) and Student’s t-test (bar graphs). Fig. S6 shows a similar characterization of the other lines of NRXN1-mutant ES cells.