Figure 5.
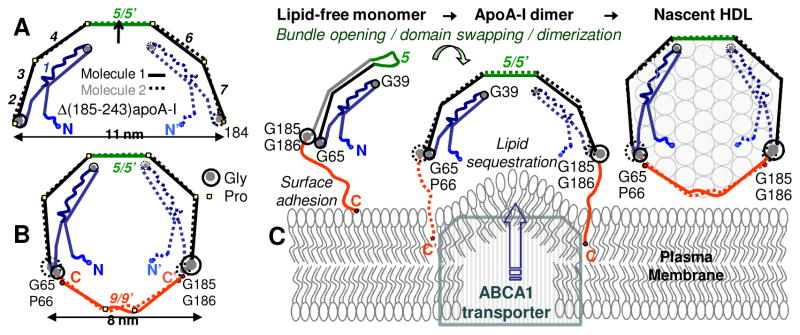
Crystal structure of the C-terminally truncated free apoA-I provides new insights into HDL biogenesis. (A) Cartoon drawn to scale shows x-ray crystal structure of Δ(185-243)apoA-I [34] (PDB ID: 3R2P). Two antiparallel dimer-forming molecules are related via the 2-fold symmetry axis passing through the middle of repeat 5. Proline-induced helical kinks in these two molecules are in register, conferring the semi-circular dimer shape whose curvature is commensurate with that of HDL. Fragment 1-184 contains seven 11/22-mer sequence repeats (numbers in italics). Straight segments represent α-helices. Critical Gly residues are indicated. (B) Proposed conformation of full-length apoA-I on small HDL [88]. The model was derived from the crystal structure in (A) by tightening the Pro-induced kinks, which allows the double-belt closure via the 185-243 fragments (red). (C) Proposed interaction mechanism of full-length apoA-I with the plasma membrane and generation of nascent HDL. Protein dimerization and insertion of the hydrophobic flexible C-terminal tails into the membrane are crucial in this process [34], which is also proposed to involve apoA-I interactions with ABCA1 transporter [68]. Figure modified from [88].
