Figure 2.
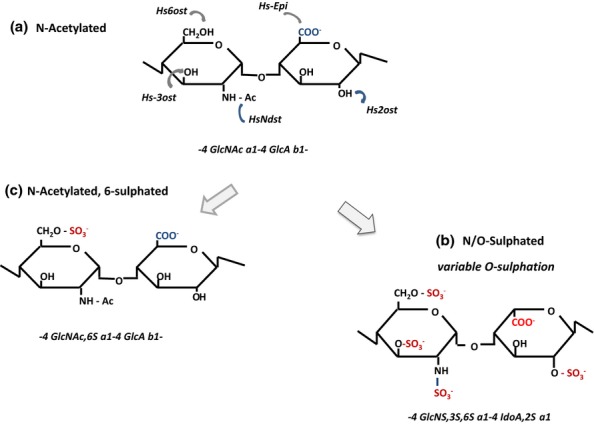
Enzymatic modifications in the biosynthesis of heparan sulphate (HS). The N-acetylated repeat disaccharide unit (a) in the HS precursor, heparan, is converted to HS by a series of modification enzymes (HS-MEs) that act in the following order: NDST, N-deactylase/N-sulphotransferase; C-5 epimerase (converts GlcA to IdoA); 2OST, 2-O-sulphotransferase; 6OST, 6-O-sulphotransferase; and 3OST, 3-O-sulphotransferase. The sequential actions of these enzymes produce a fully modified disaccharide (b) that contains IdoA and sulphate groups at all potential sites of modification. However, the modifications are incomplete at each stage, generally clustered in domains, and give rise to considerable variability in the structure of HS. Extensive regions of the heparan chain remain unmodified. S domains are formed by repeat GlcNS-IdoA, 2S units modified to varying degrees by sulphation at C6 and occasionally at C3. GlcNAc residues may be a target for 6OSTs when positioned next to an N-sulphated unit. As a consequence of this restriction, GlcNAc,6S (c) is found only in (NA)/NS regions of HS.
