Figure 3.
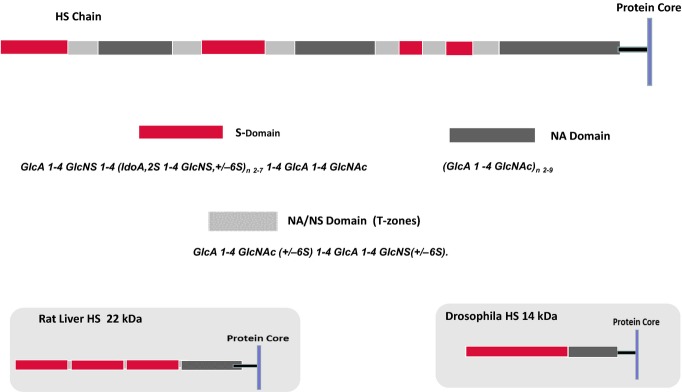
Domain structure of heparan sulphate (HS). The models illustrate a typical HS species from mammalian cells, rat liver and Drosophila. Mammalian HS is an ordered structure composed of an alternating arrangement of hypervariable sulphated regions [S- and N-acetylated (NA)/NS domains] and non-sulphated regions (NA domains) spaced in a fairly regular manner along the polymer; chain lengths vary from about 50 to 200 disaccharide units. An internal NA domain of approximately 10 disaccharides is contiguous with the glycosaminoglycan–protein linkage sequence. An S domain, often highly sulphated, is common at the distal, non-reducing end of the chain. Rat liver HS is a notable exception to the general design of mammalian HS species; it is an asymmetric structure with three, closely spaced S domains arranged towards the chain periphery but with retention of the internal, non-sulphated NA domain. HS synthesized by Drosophila is a relatively short, two-domain polymer in which a core NA sequence is connected to a longer, heparin-like distal region (Kusche-Gullberg et al. 2012). HS thus appears to have acquired a more complex structure during the course of evolution with an extension of chain length accompanied by the emergence of internal sulphated regions but with retention of the core NA domain.
