Figure 6.
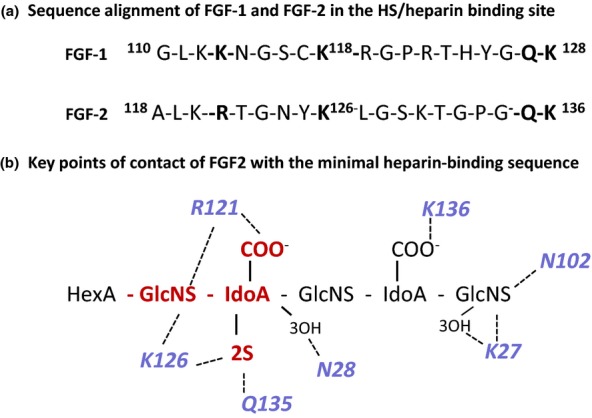
The interaction of FGF2 and FGF1 with heparin. (a) Sequence alignment of FGF2 and FGF1 in the main heparin/heparan sulphate binding sequence; conserved residues involved in heparin binding are in bold text. (b) Schematic diagram of the main FGF2 heparin contacts in the co-crystal FGF2 heparin complex in Figure 4. The GlcNS-IdoA,2S sequence (red) interacts with a high affinity subsite in FGF2; R121 are K126 are critical residues in this site. The predominant interactions are electrostatic, but Asn (N28 and N102) and Gln (Q131) participate in important H-bonds with the bound heparin. In the crystal structure, the IdoA,2S residue in the high-affinity site is in 1C4 chair conformation and the non-sulphated IdoA is in the 2SO skew boat conformer. For simplicity, non-interacting 6-O-sulphate groups on the amino sugars are not shown. FGF, fibroblast growth factors.
