Figure 12.
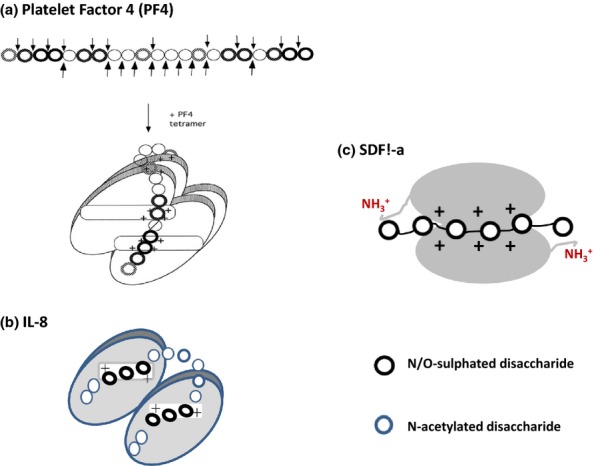
Models of CXCL chemokines in complexes with heparan sulphate (HS) and heparin. The models proposed for HS in complexes with PF4 and IL-8 are based on the structures of chemokine-binding domains in HS protected from degradation by heparinase enzymes (see text for details). In PF4, the S domains run perpendicular to the alpha-helices but adopt a parallel orientation to the alpha-helices in IL-8. The alpha-helices in SDF1-α are not involved in HS–heparin binding. Molecular docking reveals that heparin (dp12) binds along a positively charged ‘crevasse'at the interface of the SDF1-a dimer and then extends to the N-terminal lysines in each monomer (Sadir et al. 2001). Ref. PF4 model in (a): This research was originally published in Journal of Biological Chemistry. Authors: Sally E. Stringer and John T. Gallagher Title: Specific Binding of the Chemokine Platelet Factor 4 to Heparan Sulfate. J. Biol. Chem. (1997) 272, 20508–20514 © the American Society for Biochemistry and Molecular Biology.”
