Figure 14.
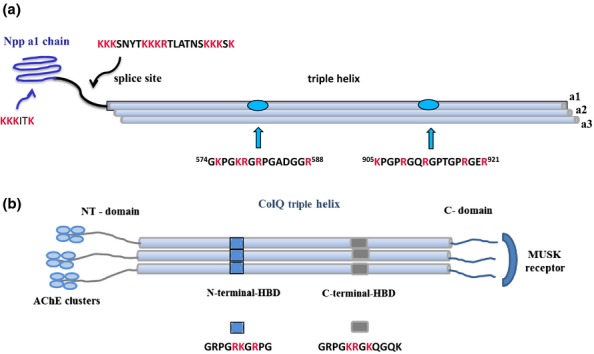
(a) Heparan sulphate (HS)/heparin binding sites in the N-terminal domain (NTD) and helical region of the α1 chain of collagen XI. Heparan sulphate-/heparin-binding regions in collagen α1(XI)-chain are found in the 223-residue globular Npp domain in the form of an XBBBXXBX CW motif, in the highly basic variable region, and in two sites in the major triple helix including a similar sequence to the 905–921 residue sequence present in the collagen type α1(V)-chain. The CW motif in the Npp domain is also present in collagen α1(V). The NTD of the α1-chain is retained in the collagen XI heterotrimeric triple helix and projects from the surface of the collagen fibril. The NTDs of the α2- and α3-chains are rapidly removed by proteolysis before assembly of the collagen XI monomer into fibrils. (b) Schematic diagram of the binding sites in synaptic collagen Q. Collagen Q is found only in the neuro-muscular synapse. It contains two (XBBXBX) CW motifs in the triple helix that interact with perlecan HS in the synaptic cleft. The non-collagenous C-terminal region binds to the muscle-specific Musk receptor. The three non-helical N-terminal regions bind four AchE subunits in an asymmetric A12/Q complex that degrades Ach and controls the strength and duration of synaptic transmission.
