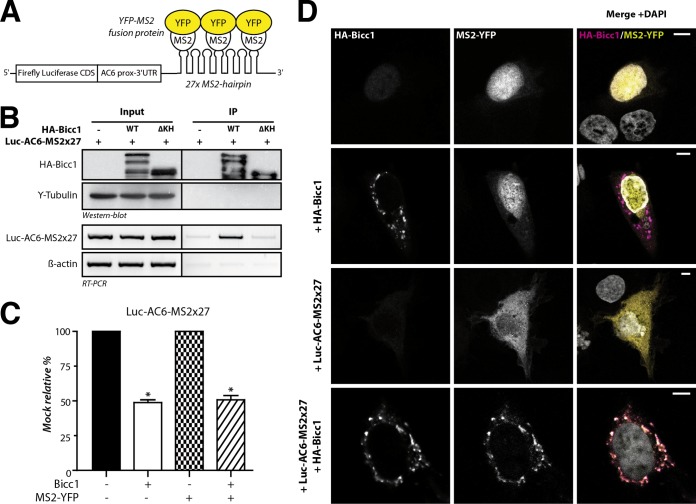
FIG 2.
Bicc1 concentrates an associated reporter mRNA in cytoplasmic foci. (A) Principle of the MS2-YFP colocalization assay. The 3′ extremity of the Luc-AC6 reporter mRNA was fused to 27 MS2 hairpins, which constitute multiple binding sites for the MS2 protein fused to YFP. CDS, coding sequence; prox, proximal. (B) RNA coimmunoprecipitation. The Luc-AC6-MS2×27 reporter mRNA was expressed in HEK293T cells together with HA-Bicc1 or empty vector. After HA immunoprecipitation (IP), the various fractions were analyzed by Western blotting and by RT-PCR. Five percent of the total extract was used as the input. The β-actin mRNA was used as a negative control for the RT-PCR. HA-Bicc1 lacking all KH and KH-like domains (ΔKH) was used as an additional negative control for RNA-binding specificity. (C) Cotransfection of the fluorescent YFP-MS2 fusion protein does not affect the silencing of the Luc-AC6-MS2×27 reporter by HA-Bicc1. β-Galactosidase was used as a control for normalization, and the data represent the percent expression relative to that of a mock-treated control. Error bars show SEMs. *, P < 0.005. (D) Localization by indirect immunofluorescence staining of the Luc-AC6-MS2×27 reporter mRNA and HA-Bicc1 in COS-1 cells. The MS2-tagged mRNA was detected by the relocalization of fluorescent MS2-YFP fusion protein, which binds the MS2 RNA hairpins. Bars, 5 μm.