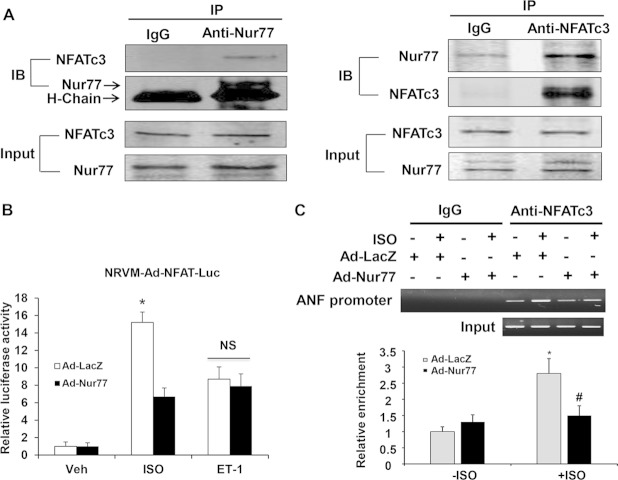
FIG 9.
Functional interaction of Nur77 with NFATc3. (A) Myocytes were treated with ISO (10 μmol/liter) for 3 h. The nuclear fraction of cardiomyocytes was isolated and then subjected to immunoprecipitation with either normal IgG, anti-NFATc3, or anti-Nur77 antibody. Immunocomplexes were separated by 10% SDS-PAGE and immunoblotted with either anti-Nur77 or NFATc3 antibody. (B) Myocytes were transduced with either Ad-LacZ or Ad-Nur77 (MOI, 30). At 24 h after transduction, myocytes were then transduced with Ad-NFAT-Luc (MOI, 30). At 24 h after transduction, myocytes were starved and stimulated with ISO (10 μmol/liter) or ET-1 (200 nmol/liter) for 24 h, and luciferase assays were performed (n = 5). *, P < 0.05 versus Ad-LacZ with Veh or Ad-Nur77 with ISO. (C) Nur77 inhibits the binding activity of NFATc3 to the ANF promoter. NRCMs were infected with Ad-Nur77 or Ad-LacZ for 48 h and then treated with vehicle or 10 μmol/liter ISO for 3 h. PCR analysis of sheared DNA from control and adenovirus-infected cells before immunoprecipitation (input) and after chromatin immunoprecipitation (ChIP) with antibody directed against NFATc3 and quantitative analysis of ChIP results from three independent experiments are shown. *, P < 0.05 versus Ad-LacZ without ISO; #, P < 0.05 versus Ad-LacZ with ISO.