Figure 1. The L-tyrosine biosynthetic pathway engineering strategy.
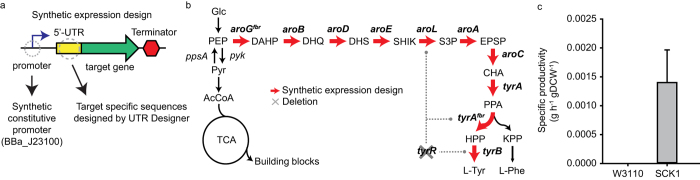
(a) Each gene was under the control of synthetic expression design that substitutes native promoter and 5′-UTR with synthetic constitutive promoter and designed 5′-UTR specific to target gene. (b) Dashed lines indicate feedback regulation, ‘X’ denotes deletion of the tyrR gene, and thick red arrows represent overexpression of genes in the L-tyrosine synthetic pathway. Abbreviations: Glc, glucose; PEP, phosphoenolpyruvate; Pyr, pyruvate; AcCoA, acetyl-CoA; DAHP, 3-deoxy-D-arabino-heptulosonate-7-phosphate; DHQ, 3-dehydroquinate; DHS, 3-dehydroshikimate; SHIK, shikimate; S3P, shikimate-3-phosphate; EPSP, 5-enolpyruvylshikimate-3-phosphate; CHA, chorismate; PPA, prephenate; HPP, 3-hydroxyphenylpyruvate; L-tyr, L-tyrosine; KPP, keto-phenylpyruvate; L-Phe, L-phenylalanine. (c) Comparison of L-tyrosine productivity of wild-type (W3110) and rationally engineered (SCK1) E. coli strain. The specific productivity of L-tyrosine was increased to 0.0014 g/h/g DCW in the SCK1 strain while the wild type strain did not produce L-tyrosine. The y-axis represents specific productivity of L-tyrosine (g/h/g DCW) in each strain. Each point and error bar indicates means and standard deviations between measurements from biological triplicate cultures.
