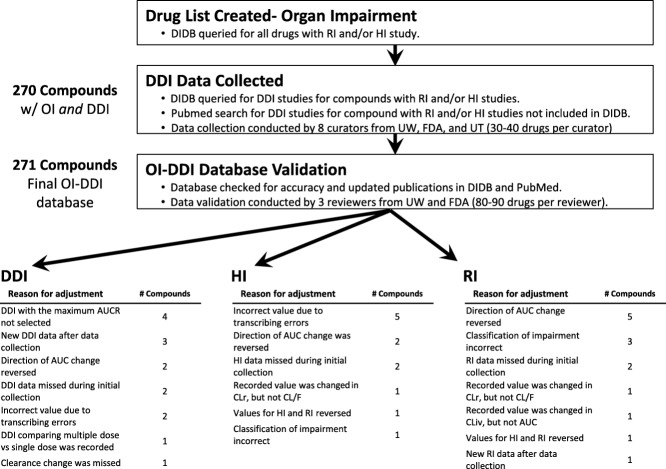
Figure 1.
Flowchart of the database construction and validation processes. AUC, area under the concentration-time curve; CL, clearance; CLiv, clearance after intravenous administration; CLr, renal clearance; DDI, drug–drug interaction; DIDB, Drug Interaction Database; F, absolute bioavailability; FDA, Food and Drug Administration; HI, hepatic impairment; OI, organ impairment; RI, renal impairment; UT, University of Tokyo; UW, University of Washington.