Abstract
Background
The role of multi-modality therapy in stage IIIB NSCLC remains inadequately studied. Although chemoradiation is currently the mainstay of treatment, randomized trials evaluating surgery are lacking and resection is offered selectively.
Methods
Data of clinical stage IIIB NSCLC patients (T4N2 or any N3) undergoing definitive multimodality therapy were obtained from the National Cancer Database (NCDB). Multivariable Cox regression models were fitted to evaluate variables influencing overall survival (OS).
Results
From 1998-2010, 7,459 clinical stage IIIB NSCLC patients were treated with definitive chemoradiation (CR group), while 1,714 patients underwent chemotherapy, radiation, and surgery in any sequence (CRS group). CRS patients were more likely to be younger, Caucasian, and have slightly smaller tumors (all p < 0.01). There was no difference in Charlson Comorbidity Index (CCI) between the groups (p = 0.5).
In the CRS group, 79% of patients received neoadjuvant therapy. Thirty-day surgical mortality was 3%. Factors associated with improved OS in multivariate analysis included younger age, female gender, decreased CCI, smaller tumor size, and surgical resection (HR 0.57, 95% CI 0.52-0.63). Among patients treated with surgery, incomplete resection was associated with decreased OS (HR 1.52, 95% CI 1.20-1.92). Median OS was longer in CRS patients (25.9 months vs. 16.3 months, p<0.001). Propensity matched analysis on 631 patient-pairs treated with CRS vs. CR confirmed these findings (median OS = 28.9 vs. 17.2 months, p<0.001).
Conclusions
Surgical resection as a part of multimodality therapy may be associated with improved overall survival in highly selected patients with stage IIIB NSCLC. Multidisciplinary evaluation of these patients is critical.
Introduction
Lung cancer is the second most common malignancy and the most common cause of cancer death in the United States.1 For patients with stage IIIB non-small cell lung cancer (NSCLC)(T4N2 or anyN3), chemoradiation has traditionally formed the mainstay of treatment with 5-year overall survival rates of approximately 10%.2-4
Several studies have further examined the role of multimodality therapy in this patient population. Southwest Oncology Group (SWOG) 8805 was a phase II study of concurrent cisplatin and etoposide plus mediastinal radiation followed by surgery for the treatment of stage IIIA (N2) and selected stage IIIB NSCLC patients.5 In the stage IIIB cohort, the authors noted an 80% resectability rate following induction chemoradiation and a 3-year overall survival (OS) rate of 24%. This compared favorably to additional phase II studies evaluating similar patients treated with chemotherapy and radiation.6 Although intriguing, there have since been no randomized studies to address the utility of surgery in patients with stage IIIB disease and its use appears to be sporadic with poorly defined criteria.
The National Cancer Database (NCDB) is a joint program developed in 1989 by the Commission on Cancer, the American College of Surgeons, and the American Cancer Society. Data is submitted by more than 1,500 accredited cancers centers across the United States, and it captures approximately 70% of all new cancer cases diagnosed in the U.S. annually. We queried the NCDB to better understand the utilization of surgery for stage IIIB NSCLC in the U.S. and evaluate its efficacy for selected patients.
Patients and Methods
For patients treated between 1998 and 2010, de-identified patient information was abstracted from the NCDB participant user file for those with clinical stage IIIB NSCLC (T4N2 or any N3 according to the 7th edition AJCC staging manual) who received either definitive chemotherapy and radiation in any order (CR group) or a combination of chemotherapy, radiation, and surgery in any order (CRS group). Patients who did not receive either one of these 2 treatment plans or those who received only chemotherapy and sub-standard doses of radiotherapy (less than 60 Gy) were excluded. There was no minimum radiation dose for patients in the surgical cohort. In addition, patients in either group who received > 100 Gy of radiation as recorded in the NCDB were excluded as well, as this was felt to reflect a likely inaccuracy in data acquisition. Patients who received only palliative treatment (as coded in the database) were excluded. Because we limited this study to the current AJCC definition of stage IIIB disease, patients with T4N0 or T4N1 tumors were not included.
Information regarding patient- and tumor-related variables, treatment details, and short- and long-term outcomes were extracted from the database. Using information on race, income, and population size of the area from which a patient presented, we created dichotomized groups in which a patient was either Caucasian or not Caucasian, had an annual income less than or greater than $35,000, and presented from a rural location (regional population less than 250,000) or an urban location, respectively. The Charlson/Deyo score was used as a measure of comorbidity. It was categorized as 0, 1, or ≥ to 2. The NCDB combines those with scores of 2 or greater into a single group, as very few patients have scores greater than two. Treatment facilities were classified as community cancer programs, comprehensive community cancer programs, and academic/research centers. For the analysis, community cancer programs and comprehensive community cancer programs were categorized as non-academic centers. Last known vital status and the time between diagnosis and the follow-up date were used to determine survival.
All analyses were performed using SPSS 21.0 (SPSS 21.0 for Windows, SPSS Inc, Chicago, IL). Descriptive statistics were expressed as means +/- standard deviation unless otherwise specified. Independent samples t tests and one-way ANOVA were used to compare continuous variables. Chi-square tests were used to compare categorical data. Overall survival was estimated by the Kaplan-Meier method. Multivariate Cox regression models were fitted to evaluate variables influencing overall survival (OS). Factors accounted for in the multivariate analysis include: age, gender, race, facility type (academic vs. non-academic), income, urban location, Charlson score, tumor size, surgical resection, and N2 vs. N3 status. Propensity matching of patients in our initial analysis using a logistic regression method was used to identify two comparable groups of patients undergoing chemoradiation only versus chemoradiation and surgery. Patients in this propensity matched analysis were matched for age, facility type (academic vs. non-academic), gender, race, income, tumor size, Charlson score, and clinical N status (N2 vs. N3). For all analyses, p-values less than 0.05 were considered statistically significant.
Results
From 1998 to 2010, 9,173 patients with clinical stage IIIB NSCLC meeting the pre-determined inclusion criteria were identified in the database. This included 7,459 patients treated with chemoradiation (CR group) and 1,714 treated with chemotherapy, radiation, and surgery in any order (CRS group) (Figure 1). CRS patients were younger, more likely to be Caucasian, have higher income, and receive treatment at academic centers (Table 1). Those in the CRS group were also more likely to have smaller tumors (49.2 vs. 52.6mm, p=0.004) and less likely to have N3 disease (37% vs. 58%, p<0.001). The Charlson/Deyo comorbidity scores were similar between the groups (p=0.5).
Figure 1.
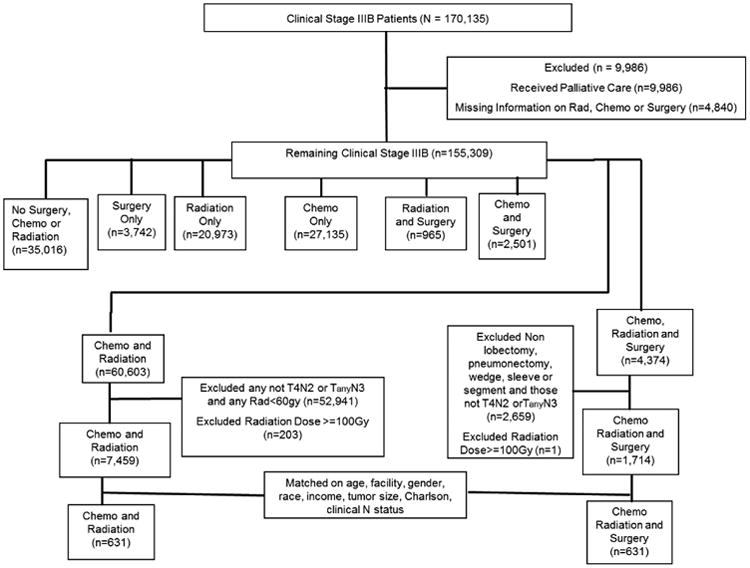
Consort diagram for 170,135 patients with stage IIIB NSCLC identified in the National Cancer Database.
Table 1.
Demographics and clinical characteristics of patients with stage IIIB NSCLC – Continuous variables are displayed as mean +/- standard deviation. Categorical variables are displayed as number (% total).
| Patient Characteristics | Chemoradiation Alone (CR) n= 7,459 | Chemoradiation Plus Surgery (CRS) n=1,714 | p-Value | |
|---|---|---|---|---|
| Age at diagnosis (years) | 64.1 +/- 10.4 | 59.7 +/- 10.5 | <0.001 | |
| Male Gender | 4,481 (60%) | 988 (58%) | 0.067 | |
| Caucasian | 6,259 (84%) | 1,512 (88%) | <0.001 | |
| Academic Center | 1,865 (25%) | 654 (39%) | <0.001 | |
| Annual Income > $35,000 | 4,707 (63%) | 1,205 (70%) | <0.001 | |
| Urban Population Area | 4,346 (58%) | 1,144 (67%) | <0.001 | |
| Charlson/Deyo Score (CCI) | 0 | 5,153 (69%) | 794 (70%) | 0.510 |
| 1 | 1,741 (23%) | 265 (23%) | ||
| 2 | 565 (8%) | 75 (7%) | ||
| Tumor Size (mm) | 52.6 +/- 42.1 | 49.2 +/- 36.5 | 0.004 | |
| AJCC Clinical N Stage | 2 | 2,905 (42%) | 978 (63%) | <0.001 |
| 3 | 4,082 (58%) | 565 (37%) | ||
Multi-agent chemotherapy predominated in both groups, while CR patients received a slightly higher dose of radiation (median dose = 65.0 vs. 59.4 Gy) (Table 2). In patients undergoing surgery, 79% of patients received neoadjuvant chemotherapy or radiation or a combination of both. Among surgical patients, complete resection was achieved in 71%. Lobectomy was performed in 60% of cases, and thirty-day operative mortality was 3%.
Table 2.
Treatment details in patients with stage IIIB NSCLC - Continuous variables are displayed as median (interquartile range). Categorical variables are displayed as number (% total).
| Treatment Information | CR Group n=7,459 | CRS Group n=1,714 | p-Value | |
|---|---|---|---|---|
| Radiation Sequence | Pre-op | n/a | 903 (53%) | n/a |
| Post-op | 700 (41%) | |||
| Both | 33 (2%) | |||
| Other / Unknown | 78 (4%) | |||
| Median Total Radiation Dose – cGy (Interquartile range) | 6500 (6300-6840) | 5940 (5040-6480) | <0.001 | |
| Chemotherapy Sequence (n=709) | Pre-op | n/a | 366 (52%) | n/a |
| Post-op | 266 (37%) | |||
| Both | 76 (11%) | |||
| Type of Chemotherapy | Multi-agent | 6,602 (86%) | 1,455 (85%) | <0.001 |
| Single-agent | 457 (6%) | 78 (5%) | ||
| Unknown | 603 (8%) | 181 (11%) | ||
| Surgical Margins | R0 | n/a | 1,218 (71%) | n/a |
| R1/R2 | 298 (17%) | |||
| Unknown | 198 (12%) | |||
| Surgery Type | Lobectomy | n/a | 1,021 (60%) | n/a |
| Pneumonectomy | 350 (20%) | |||
| Wedge/segmentectomy | 343 (20%) |
In an unmatched comparison, median OS was longer in the CRS group (25.9 vs. 16.3 months, p<0.001) (Figure 2). In a subset analysis of patients with N3 disease, median OS was again longer in CRS patients (25.2 vs. 16.4 months, p<0.001). For CRS patients with N3 disease, 5-year OS following resection was 26.1%. Multivariate Cox regression analysis was used to identify variables independently associated long-term mortality. Factors associated with higher mortality risk included increasing age, male gender, higher comorbidity score, and larger tumor size. Surgery was independently associated with improved OS (HR 0.57, 95% CI = 0.52-0.62, p<0.001) (Table 3). For patients undergoing surgery, incomplete resection (R1/R2) was associated with decreased OS (HR 1.51, 95% CI = 1.19-1.91, p=0.001).
Figure 2. Kaplan-Meier curve for overall survival in patients receiving chemoradiation versus chemoradiation plus surgery (p<0.001).
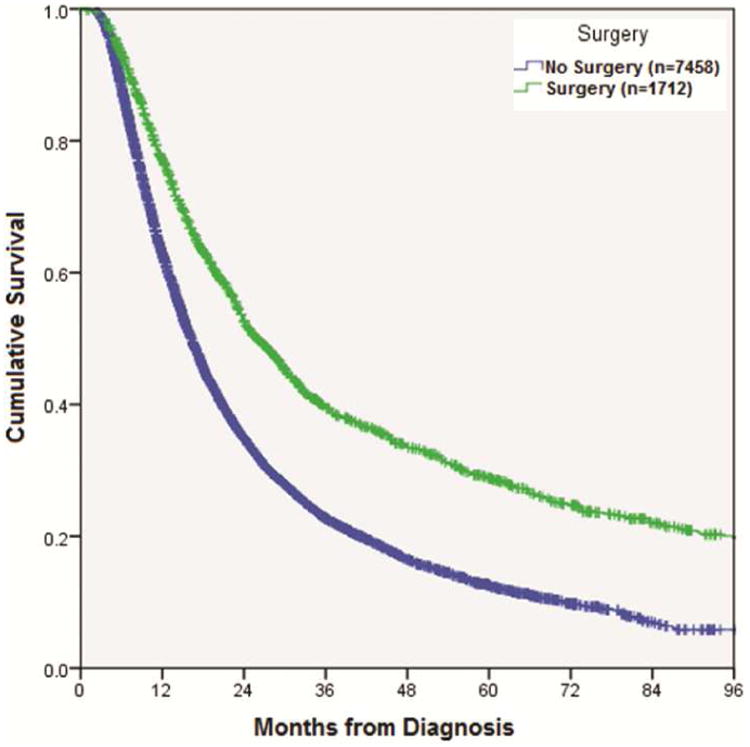
Table 3.
Results of multivariable Cox regression analysis identifying variables associated with long-term mortality in all patients with stage IIIB NSCLC.
| Patient and Treatment Variable | Hazard ratio (HR) with 95% Confidence Interval (CI) | p-Value |
|---|---|---|
| Age | 1.01 (1.01-1.01) | <0.001 |
| Male gender | 1.18 (1.11-1.25) | <0.001 |
| Population > 250,000 | 0.94 (0.88-0.99) | 0.032 |
| Charlson Score = 1 (reference =0) | 1.16 (1.09-1.25) | <0.001 |
| Charlson Score = 2 (reference=0) | 1.28 (1.15-1.42) | <0.001 |
| Tumor Size (mm) | 1.00 (1.00-1.01) | <0.001 |
| Surgical Resection | 0.57 (0.52-0.62) | <0.001 |
Propensity matching of patients in the initial IIIB cohort identified 631 matched pairs who underwent CR vs. CRS (Table 4). Following matching, patients in the CRS group continued to show improved survival compared with those in the CR group (median overall survival = 28.9 months vs. 17.2 months, p<0.001) (Figure 3).
Table 4.
Propensity score matching of patients undergoing CR vs. CRS led to 631 matched pairs. Continuous variables are displayed as mean +/- standard deviation. Categorical variables are displayed as number (% total).
| Patient Characteristics | CR Group n= 631 | CRS Group n=631 | p-Value | |
|---|---|---|---|---|
| Age at diagnosis (years) | 62.0 +/- 10.4 | 62.1 +/- 9.4 | 0.94 | |
| Male Gender | 378 (60%) | 360 (57%) | 0.33 | |
| Caucasian | 534 (85%) | 541 (86%) | 0.63 | |
| Academic Center | 212 (34%) | 198 (31%) | 0.43 | |
| Annual Income > $35,000 | 419 (66%) | 422 (67%) | 0.90 | |
| Urban Population Area | 387 (61%) | 409 (65%) | 0.22 | |
| Charlson/Deyo Score (CCI) | 0 | 454 (72%) | 429 (68%) | 0.27 |
| 1 | 136 (22%) | 151 (24%) | ||
| 2 | 41 (6%) | 51 (8%) | ||
| Tumor Size (mm) | 49.5 +/- 25.2 | 47.9 +/- 27.0 | 0.28 | |
| AJCC Clinical N Stage | 2 | 348 (55%) | 343 (54%) | 0.82 |
| 3 | 283 (45%) | 288 (46%) | ||
| Median Overall Survival (months) | 17.2 +/- 1.0 | 28.9 +/- 1.8 | <0.001 | |
Figure 3.
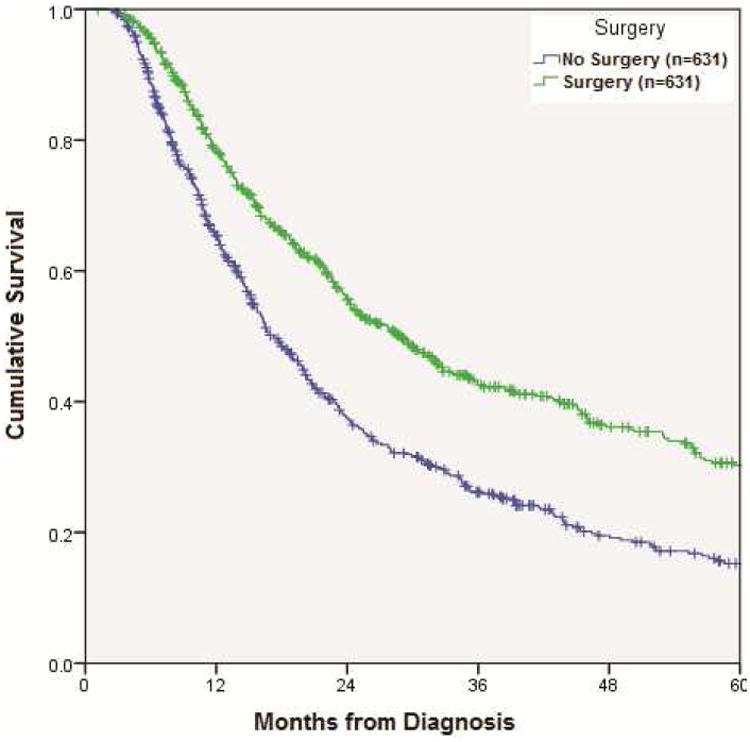
Kaplan-Meier curve for overall survival in CR vs. CRS patients after propensity score matching of 631 patient pairs (p<0.001).
Of the 1,243 patients with data available regarding the timing of chemoradiation in relation to surgical resection, 258 patients (21%) underwent initial surgical resection followed by adjuvant chemoradiation (Table 5). There was no significant difference in survival between those patients treated with neo-adjuvant chemoradiation followed by surgical resection versus those undergoing resection followed by adjuvant chemoradiation (median overall survival = 30.0 months vs. 26.6 months, p=0.06). Notably, despite larger average tumor size, the neoadjuvant group had a higher rate of R0 resection (83% vs. 56%, p<0.001).
Table 5.
Clinical characteristics and outcome data of patients with stage IIIB NSCLC treated with neoadjuvant chemoradiation followed by surgical resection versus primary resection followed by adjuvant chemoradiation – Continuous variables are displayed as mean +/- standard deviation. Categorical variables are displayed as number (% total).
| Patient Characteristics | Neoadjuvant treatment n= 985 | Adjuvant treatment n=258 | p-Value | |
|---|---|---|---|---|
| Age at diagnosis (years) | 59.0 +/- 10.0 | 62.3 +/- 10.0 | <0.001 | |
| Male Gender | 557 (57%) | 142 (55%) | 0.67 | |
| Caucasian | 876 (89%) | 216 (83%) | 0.02 | |
| Academic Center | 412 (43%) | 73 (29%) | <0.001 | |
| Annual Income > $35,000 | 718 (73%) | 157 (61%) | <0.001 | |
| Urban Population Area | 661 (67%) | 162 (63%) | 0.16 | |
| Charlson/Deyo Score (CCI) | 0 | 515 (73%) | 151 (58%) | <0.001 |
| 1 | 156 (22%) | 76 (29%) | ||
| 2 | 30 (4%) | 32 (12%) | ||
| Tumor Size (mm) | 53.5 +/- 35.7 | 38.3 +/- 26.0 | <0.001 | |
| AJCC Clinical N Stage | 2 | 569 (63%) | 148 (64%) | 0.82 |
| 3 | 338 (37%) | 85 (36%) | ||
| Negative Margin (R0 resection) | 815 (83%) | 146 (56%) | <0.001 | |
| Median Overall Survival (months) | 30.0 +/- 1.7 | 26.6 +/- 2.8 | 0.06 | |
Comment
Chemoradiotherapy has traditionally been the mainstay of treatment for locally advanced NSCLC, however long term survival remains limited. Despite aggressive therapy, median overall survival for patients treated with optimal chemoradiation ranges from 15-23 months, with a 3-year overall survival of less than 30%.6-8
As a result, several previous authors have explored the utility of surgical resection in carefully selected patients with stage IIIB disease. Retrospective series have found a 3-year overall survival of 42-56% in patients with stage IIIB disease undergoing multimodality therapy including surgical resection, and a favorable response to induction therapy appears to be a significant predictor of survival.9-10 A series of 40 patients with stage IIIB NSCLC by Grunenwald et al noted that those who underwent complete resection and had no lymphatic metastases on final pathology had a 5-year overall survival of 42%.11
SWOG 8805 was initiated as a feasibility study of patients undergoing surgical resection following induction chemoradiation for the treatment of stage IIIA and IIIB NSCLC.12 Preliminary analysis of the first 75 eligible patients indicated that complete resection was feasible in 73% of cases. In addition, combined modality therapy appeared to be well tolerated with an operative mortality of 6% and a low rate of major morbidity.
Based on these feasibility data, SWOG 8805 was continued as a prospective phase II trial to evaluate the efficacy of surgical resection as part of multimodality therapy in patients with IIIA and IIIB NSCLC.5,13 Eligible stage IIIB patients had either invasion into mediastinal structures (T4) or biopsy-positive contralateral mediastinal or supraclavicular lymph nodes (N3). Once enrolled, patients were treated with an induction regimen including two cycles of cisplatin plus etoposide and concurrent chest radiotherapy (45 Gy) to the primary tumor and mediastinal lymph nodes. In stage IIIB patients that went on to surgery, 80% underwent complete resection and the absence of tumor in the mediastinal lymph nodes at the time of surgery was a strong predictor of survival. Overall survival in the IIIB cohort was 24% at 3 years, which was similar to IIIA patients treated with the same regimen (27% 3-year overall survival).
In order to draw a more direct comparison to patients treated only with chemoradiation, SWOG conducted a follow-up phase II study (SWOG 9019) of 50 patients with stage IIIB NSCLC treated with concurrent cisplatin and etoposide and chest radiotherapy using the same entry criteria as SWOG 8805.6 Once enrolled, patients were treated with the same initial chemoradiation regimen (cisplatin / etoposide and 45 Gy of concurrent radiation). In the absence of disease progression, patients went on to receive an additional 2 cycles of chemotherapy and radiation up to a total dose of 61 Gy. At three years, overall survival appeared to be somewhat inferior to the historical results from the previous study (17% vs. 24% in patients from SWOG 8805).
In 2009, a similar prospective phase II study of stage IIIB NSCLC patients treated with neoadjuvant chemoradiation followed by surgery was conducted by Stupp et al.14 Following induction therapy, complete resection was feasible in 59% of patients. Pathological mediastinal downstaging was seen in 39% of patients with lymph node involvement at the time of enrollment. After a median follow-up of 58 months, 3- and 5-year overall survival was 47% and 40%, respectively.
The results from our current study further suggest that surgical resection may be beneficial as part of multimodality therapy for highly selected patients with stage IIIB NSCLC. Outcomes in our series suggest that surgical resection is associated with greater likelihood of overall survival (HR = 0.57, p< 0.001). We noted that median overall survival was longer in CRS patients (25.9 months vs. 16.3 months). This is longer than median survival in surgical patients from SWOG 8805 (17 months) but similar to outcomes in the more recent phase II trial by Stupp (29 months). Likewise, the median survival of CR group patients in our study (16.3 months) was comparable to similarly treated patients in SWOG 9019 (median overall survival = 15 months). Interestingly, the benefit associated with resection was not limited to patients with T4 disease. In our subset analysis of patients with clinical N3 tumors, surgery was associated with a prolonged median survival (25.2 months) compared with N3 patients in the CR group (16.4 months).
Although many patients underwent fairly aggressive induction therapy with dual agent chemotherapy and median radiation dose of 59.4 Gy, surgical mortality was acceptably low (3%) indicating that surgery was indeed safe in selected patients. In addition, a complete resection was obtained in upwards of 70% of cases and only 20% of patients required a pneumonectomy.
Most established treatment protocols for locally advanced NSCLC (stage IIIA) would favor neoadjuvant chemoradiation, so the choice for initial surgical resection in 21% of our stage IIIB population in intriguing. Upon closer examination of the 258 patients receiving surgery followed by adjuvant chemoradiation, 178 (69%) had T4 tumors while 85 (36%) had N3 nodal status. Although it is possible that the decision for surgical resection was made based on T4 status in most instances, the true impetus for primary resection in these patients remains unclear.
When considering these data it is important to note that the most recent update of the AJCC staging system for NSCLC has altered the classification of stage IIIB disease. According to the 6th edition, patients with T4N0-3 and anyTN3 were all categorized as stage IIIB disease. In the most recent 7th edition published in 2010, T4N0-1 disease is now included in stage IIIA and only patients having T4N2 or anyTN3 remain in the stage IIIB subgrouping.2 Thus, our study population likely comprises a cohort with somewhat more advanced disease than previous trials evaluating stage IIIB patients.
The question of patient selection for resection is difficult and has been raised repeatedly in the context of stage III NSCLC. Significant response to induction chemotherapy, both in the form of mediastinal downstaging and complete pathologic response, has been associated with improved survival in patients with stage III NSCLC undergoing trimodality therapy.15-17 However, pathologic downstaging and complete response are difficult to predict clinically and one may make the argument that resection was retrospectively unnecessary in those with complete response. Staging modalities such as PET/CT tend to underestimate the degree of pathologic response, so their utility in patient selection may be limited.15,18 Therefore it is likely reasonable to consider surgical resection as part of multimodality therapy in patients with significant clinical response, particularly those demonstrating clinical mediastinal downstaging or resolution. However, given the poor sensitivity of current non-invasive re-staging modalities, we recommend that all patients with stage IIIB disease be discussed in a multi-disciplinary format in order to optimize outcomes.
There are several limitations to the current study. Although the NCDB contains a wealth of patient information, the data are still retrospectively reviewed and are not randomized. Selection bias in terms of which patients are offered surgical resection potentially influences long term outcomes. For instance, clinical judgment regarding the feasibility of complete surgical resection likely plays a role in patient selection. In addition, although the comorbidity index was not significantly different between the CR and CRS groups, it is still possible that unrecorded differences exist in these patient populations, particularly in regards to performance status and comorbid disease. We performed a propensity score matched analysis to minimize the effects of such bias, however, despite matching for several important variables it is still possible that more subtle differences in the CR and CRS groups remain. Certain information such as the subclassification of T4 status (multiple tumor nodules vs. invasion of adjacent structures), N3 status (supraclavicular vs. contralateral mediastinal nodes), and response to induction therapy is not available from the database and therefore cannot be analyzed. Lastly, the database cannot provide information on patients who may have been initially considered for trimodality therapy but eventually did not receive surgery either due to disease progression or complications of induction therapy.
Although chemotherapy and radiation currently comprise the standard treatment for stage IIIB non-small cell lung cancer, our analysis of data from the NCDB suggests that surgical resection may be beneficial for carefully selected patients. While the limitations of this retrospective study prevent recommendations regarding routine resection of all patients with stage IIIB disease, the results of this descriptive analysis can inform the treating physician that all patients with clinical stage IIIB disease are not identical. In advocating for a more tailored approach, these data emphasize the importance of pathologic staging of patients with clinical stage IIIB NSCLC and suggest that these patients should be discussed in a multidisciplinary setting with surgical resection potentially considered in highly selected patients.
Acknowledgments
Grant Support: Varun Puri - NIH K07CA178120, K12CA167540-02 (Paul Calabresi Award)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society. Cancer Facts and Figures 2014. Atlanta: American Cancer Society; 2014. [Google Scholar]
- 2.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7th. New York, NY: Springer; 2010. [Google Scholar]
- 3.Ramnath N, Dilling TJ, Harris LJ, Kim AW, Michaud GC, Balekian AA, Diekemper R, Detterbeck FC, Arenberg DA. Treatment of Stage III Non-small Cell Lung Cancer - Diagnosis and Management of Lung Cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. CHEST. 2013;143(5 (Suppl)):e314S–e340S. doi: 10.1378/chest.12-2360. [DOI] [PubMed] [Google Scholar]
- 4.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the TNM Stage Groupings in the Forthcoming (Seventh) Edition of the TNM Classification of Malignant Tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 5.Albain KS, Rusch VW, Crowley JJ, Rice TW, Turrisi AT, Weick JK, Lonchyna VA, Presant CA, McKenna RJ, Gandara DR, Fosmire H, Taylor SA, Stelzer KJ, Beasley KR, Livingston RB. Concurrent Cisplatin/Etoposide Plus Chest Radiotherapy Followed by Surgery for Stages IIIA (N2) and IIIB Non-Small-Cell Lung Cancer: Mature Results of Southwest Oncology Group Phase II Study 8805. J Clin Oncol. 1995;13:1880–1892. doi: 10.1200/JCO.1995.13.8.1880. [DOI] [PubMed] [Google Scholar]
- 6.Albain KS, Crowley JJ, Turrisi AT, Gandara DR, Farrar WB, Clark JI, Beasley KR, Livingston RB. Concurrent Cisplatin, Etoposide, and Chest Radiotherapy in Pathologic Stage IIIB Non-Small-Cell Lung Cancer: A Southwest Oncology Group Phase II Study, SWOG 9019. J Clin Oncol. 2002;20:3454–3460. doi: 10.1200/JCO.2002.03.055. [DOI] [PubMed] [Google Scholar]
- 7.Pujol J, Lafontaine T, Quantin X, Reme-Saumon M, Cupissol D, Khial F, Michel F. Neoadjuvant Etoposide, Ifosfamide, and Cisplatin Followed by Concomitant Thoracic Radiotherapy and Continuous Cisplatin Infusion in Stage IIIb Non-small Cell Lung Cancer. Chest. 1999;115:144–150. doi: 10.1378/chest.115.1.144. [DOI] [PubMed] [Google Scholar]
- 8.Kelly K, Chansky K, Gaspar LE, Albain KS, Jett J, Ung YC, Lau DHM, Crowley JJ, Gandara DR. Phase III Trial of Maintenance Gefitinib or Placebo After Concurrent Chemoradiotherapy and Docetaxel Consolidation in Inoperable Stage III Non-Small-Cell Lung Cancer: SWOG S0023. J Clin Oncol. 2008;26:2450–2456. doi: 10.1200/JCO.2007.14.4824. [DOI] [PubMed] [Google Scholar]
- 9.Ichinose Y, Fukuyama, Asoh H, Ushijima C, Okamoto T, Ikeda J, Okamoto J, Sakai M. Induction Chemoradiotherapy and Surgical Resection for Selected Stage IIIB Non-Small-Cell Lung Cancer. Ann Thorac Surg. 2003;76:1810–1815. doi: 10.1016/s0003-4975(03)01075-0. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Dai CH, Yu LC, Ping C, Li XQ, Shi SB, Wu JR. Results of Trimodality Therapy in Patients with Stage IIIA (N2-Bulky) and Stage IIIB Non-Small-Cell Lung Cancer. Clinical Lung Cancer. 2009;10(5):353–359. doi: 10.3816/CLC.2009.n.048. [DOI] [PubMed] [Google Scholar]
- 11.Grunenwald DH, Andre F, Pechoux CL, Girard P, Lamer C, Laplanche A, Tarayre M, Arriagada R, Chevalier TL. Benefit of surgery after chemoradiotherapy in stage IIIB (T4 and/or N3) non-small cell lung cancer. J Thorac Cardiovasc Surg. 2001;122:796–802. doi: 10.1067/mtc.2001.116472. [DOI] [PubMed] [Google Scholar]
- 12.Rusch VW, Albain KS, Crowley JJ, Rice TW, Lonchyna V, McKenna R, Livingston RB, Griffin BR, Benfield JR. Surgical resection of stage IIIA and stage IIIB non-small-cell lung cancer after concurrent induction chemoradiotherapy: A Southwest Oncology Group Trial. J Thorac Cardiovasc Surg. 1993;105:97–106. [PubMed] [Google Scholar]
- 13.Rusch VW, Albain KS, Crowley JJ, Rice TW, Lonchyna V, McKenna R, Stelzer K, Livingston RB the Southwest Oncology Group. Neoadjuvant Therapy: A Novel and Effective Treatment for Stage IIIb Non-Small Cell Lung Cancer. Ann Thorac Surg. 1994;58:290–295. doi: 10.1016/0003-4975(94)92195-4. [DOI] [PubMed] [Google Scholar]
- 14.Stupp R, Mayer M, Kann R, Weder W, Zouhair A, Betticher DC, Roth AD, Stahel RA, Majno SB, Peters S, Jost L, Furrer M, Thierstein S, Schmid RA, Hsu-Schmitz SF, Mirimanoff RO, Ris SB, Pless M. Neoadjuvant chemotherapy and radiotherapy followed by surgery in selected patients with stage IIIB non-small-cell lung cancer: a multicenter phase II trial. Lancet Oncol. 2009;10:785–793. doi: 10.1016/S1470-2045(09)70172-X. [DOI] [PubMed] [Google Scholar]
- 15.Pisters KMW, Kris MG, Gralla RJ, Zaman MB, Heelan RT, Martini N. Pathologic Complete Response in Advanced Non-Small-Cell Lung Cancer Following Preoperative Chemotherapy: Implications for the Design of Future Non-Small-Cell Lung Cancer Combined Modality Trials. J Clin Oncol. 1993;11:1757–1762. doi: 10.1200/JCO.1993.11.9.1757. [DOI] [PubMed] [Google Scholar]
- 16.Decaluwe H, De Leyn P, Vansteenkiste J, Dooms C, Van Raemdonck D, Nafteux P, Coosemans W, Lerut T. Surgical multimodality treatment for baseline resectable stage IIIA-N2 non-small cell lung cancer. Degree of mediastinal lymph node involvement and impact on survival. Eur J Cardiothorac Surg. 2009;36:433–439. doi: 10.1016/j.ejcts.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Trodella L, Granone P, Valente S, Margaritora S, Macis G, Cesario A, D'Angelillo RM, Valentini V, Corbo GM, Porziella V, Ramella S, Tonini G, Galetta D, Ciresa M, Vincenzi B, Cellini N. Neoadjuvant concurrent radiochemotherapy in locally advanced (IIIA-IIIB) non-small-cell lung cancer: long-term results according to downstaging. Ann Oncol. 2004;15:389–398. doi: 10.1093/annonc/mdh099. [DOI] [PubMed] [Google Scholar]
- 18.Thomas M, Rube C, Semik M, von Eiff M, Freitag L, Macha HN, Wagner W, Klinke F, Scheld HH, Willich N, Berdel WE, Junker K. Impact of Preoperative Bimodality Induction Including Twice-Daily Radiation on Tumor Regression and Survival in Stage III Non-Small-Cell Lung Cancer. J Clin Oncol. 1999;17:1185–1193. doi: 10.1200/JCO.1999.17.4.1185. [DOI] [PubMed] [Google Scholar]


